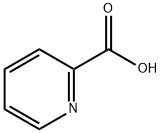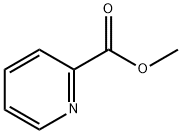
Methyl picolinate synthesis
- Product Name:Methyl picolinate
- CAS Number:2459-07-6
- Molecular formula:C7H7NO2
- Molecular Weight:137.14
Yield:2459-07-6 83%
Reaction Conditions:
with oxygen;potassium carbonate at 60; for 6 h;Catalytic behavior;Sealed tube;
Steps:
2.6. General procedure for oxidative esterification of benzylicalcohols
General procedure: A 50 ml round bottomed flask was charged with heterogeneousPd catalyst 1 (1 mol%), K2CO3(1.2 equiv.) and methanol (3 ml). Thebase was soluble in the methanol and methanol acted as bothreactant and reaction media for the present transformation. Theresulting flask was sealed with septum, evacuated and back-filledwith oxygen. After that the benzyl alcohol (1.0 equiv.) was added tothe above suspension via syringe. The reaction mixture was stirredat 60C for the time as given in Table 1 in the presence of oxy-gen balloon. After completion, the mixture was cooled to roomtemperature and catalyst was recovered by the influence of exter-nal magnet. The crude product was dissolved in ethyl acetate andwashed successively with water to remove the base. The organiclayer was dried over anhydrous MgSO4and concentrated underreduced pressure. The residue so obtained was purified by columnchromatography using eluent ethyl acetate in hexane (20:80) togive the pure desired product. The recovered catalyst was washedwith methanol, dried in vacuum and used for the subsequent runs.
References:
Verma, Sanny;Verma, Deepak;Sinha, Anil K.;Jain, Suman L. [Applied Catalysis A: General,2015,vol. 489,# 1,p. 17 - 23]

1121-60-4
515 suppliers
$10.60/10gm:
67-56-1
757 suppliers
$7.29/5ml-f

2459-07-6
239 suppliers
$5.00/1g
109-09-1
11 suppliers
$10.60/1gm:
67-56-1
757 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

2459-07-6
239 suppliers
$5.00/1g

5029-67-4
295 suppliers
$6.00/1g
67-56-1
757 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

2459-07-6
239 suppliers
$5.00/1g