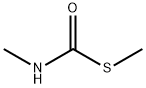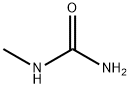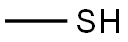
METHYL MERCAPTAN synthesis
- Product Name:METHYL MERCAPTAN
- CAS Number:74-93-1
- Molecular formula:CH4S
- Molecular Weight:48.11
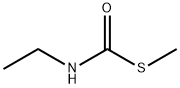
39076-43-2
0 suppliers
inquiry
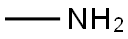
74-89-5
7 suppliers
$13.44/25ML
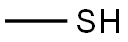
74-93-1
133 suppliers
$454.02/5MG
28145-10-0
16 suppliers
$495.63/5MG
Yield:28145-10-0 100%
Reaction Conditions:
in water at 50 - 55; for 1 h;
Steps:
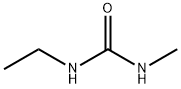
18 EXAMPLE 18; N-Ethyl-N'methyl Urea
The reaction mixture constituted by N-ethyl, S-methyl thiocarbamate (g 8.45, moles 0.071) and methylamine (g 6.6, moles 0.213; g 16.5 of 40% aqueous solution) is continuously stirred and heated to 50-55 C. The freed methanthiol is absorbed by a sodium hydroxide aqueous solution. GC monitoring shows that the reaction is completed in 1 hour. The methanthiol is completely removed from the reaction mixture through an air flow. The methylamine that is still present and the water are removed by means of a rotating evaporator, with the possible help of an azeotrope-producing solvent. g 7.24 (quantitative yield) of virtually pure (TLC, GC, GC-MS verified) N-ethyl-N-methyl urea is produced; after drying it with chloroform and subsequently crystallising it from ethylic ether, it has m.p. 50.5-51.0 C.; MS: m/e 102 (M+); 1H NMR (CDCl3), d=1.12 (t, J=7.00 Hz, 3H, CH2CH3), 2.75 (d, J=5.00 Hz, 3H, NHCH3), 3.17 (dq, J=7.00 Hz, 2H, CH2), 5.53 (enlarged s, 1H, NH).
References:
US6723875,2004,B1 Location in patent:Page column 8-9
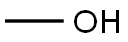
67-56-1
756 suppliers
$9.00/25ml
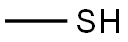
74-93-1
133 suppliers
$454.02/5MG
201230-82-2
1 suppliers
inquiry
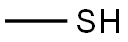
74-93-1
133 suppliers
$454.02/5MG

74-87-3
211 suppliers
$18.50/48622
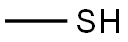
74-93-1
133 suppliers
$454.02/5MG