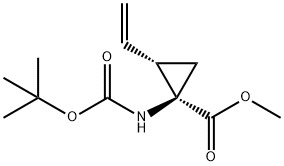
methyl ester, (1R,2S)- synthesis
- Product Name:methyl ester, (1R,2S)-
- CAS Number:159622-09-0
- Molecular formula:C12H19NO4
- Molecular Weight:241.28

67-56-1
757 suppliers
$9.00/25ml

159622-10-3
149 suppliers
$12.00/250mg

159622-09-0
34 suppliers
inquiry
Yield:159622-09-0 92%
Reaction Conditions:
with diazomethyl-trimethyl-silane in hexane;toluene at 20; for 0.5 h;
Steps:
Synthesis of (1R,2S)-13
To a solution of (1R,2S)-12 (258 mg, 1.14 mmol) in methanol(2.9 mL) and toluene (11.6 mL) was added a 0.6 Msolution of trimethylsilyldiazomethane in hexane (2.77 mL,1.66 mmol) at rt and the mixture was stirred for 30 min.After work up with acetic acid, the whole was concentratedto give the crude product, which was chromatographed onsilica gel (hexane/ethyl acetate =10/1) affording methyl(1R,2S)-13 (253 mg, 92 %) as a colorless oil, whose NMRdata matched those previously reported (Beaulieu et al.2005) and absolute stereochemistry and optical purity wasdetermined to be 99.2 % ee by HPLC analysis under thefollowing conditions: CHIRALCEL OJ-H column (particlesize 5 μm, 250 × 4.6 mm i.d.); solvent system of 1 %ethanol in hexane in 30 min, flow rate of 0.75 mL/min,25 °C, and wavelength of 200 nm, in which the (1R,2S)-isomer was eluted at a retention time (tR) of 16.4 minwhile the (1S,2R)-isomer at 18.3 min in a ratio of 99.6:0.4,respectively. An authentic sample of (1R,2S)-13 was purchasedfrom Sigma-Aldrich (Product No. ANV00168-1G),whereas an authentic sample of the (1S,2R)-isomer was preparedby esterification of the corresponding carboxylic acid purchased from Sigma-Aldrich (Product No. CDS019348-100MG) using the same procedure (TMSCHN2).[α]D25 = + 43.9 (c = 1.02, methanol) [lit. (Beaulieu et al.2005) [α]D25 = +42.8 (c = 1.00, methanol)]. 1H-NMR(200 MHz, DMSO-d6, 81:19 ratio of rotamers): δ 1.30 (1H,dd, J = 9.5, 5.0 Hz, one of 3-H2), 1.35 (0.19 × 9H, s, Me3Cfor minor rotamer), 1.37 (0.81 × 9H, s, Me3C for majorrotamer), 1.57 (1H, dd, J = 7.6, 5.0 Hz, one of 3-H2), 2.11(1H, td, J = 9.5, 7.6 Hz, 2-H), 3.60 (0.81 × 3H, s, OMefor major rotamer), 3.62 (0.19 × 3H, s, OMe for minorrotamer), 5.07 (1H, dd, J = 10.2, 2.0 Hz), 5.23 (1H, dd,J = 17.2, 2.0 Hz), 5.62 (1H, ddd, J = 17.2, 10.2, 9.5 Hz),7.34 (0.19H, br s, NH for minor rotamer), 7.68 (0.81H, brs, NH for major rotamer). 13C-NMR (50.3 MHz, DMSOd6,81:19 ratio of rotamers): δ 22.7 (3-CH2 for major rotamer),23.1 (3-CH2 for minor rotamer), 28.1 (Me3C), 32.7(2-CH for major rotamer), 33.9 (2-CH for minor rotamer),40.2 (1-C, quaternary), 51.9 (OMe), 78.2 (Me3C), 117.3(CH=CH2 for minor rotamer), 117.4 (CH=CH2 for majorrotamer), 134.3 (CH=CH2), 155.2 (CON for minor rotamer),155.5 (CON for major rotamer), 171.1 (CO2Me).
References:
Kawashima, Aki;Shu, Shuangjie;Takeda, Ryosuke;Kawamura, Akie;Sato, Tatsunori;Moriwaki, Hiroki;Wang, Jiang;Izawa, Kunisuke;Acea, Jos Luis;Soloshonok, Vadim A.;Liu, Hong [Amino Acids,2016,vol. 48,# 4,p. 973 - 986]

159622-10-3
149 suppliers
$12.00/250mg

18107-18-1
211 suppliers
$33.00/5g

159622-09-0
34 suppliers
inquiry
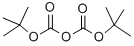
24424-99-5
839 suppliers
$13.50/25G

159622-09-0
34 suppliers
inquiry