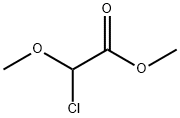
METHYL CHLORO-METHOXY ACETATE synthesis
- Product Name:METHYL CHLORO-METHOXY ACETATE
- CAS Number:13157-96-5
- Molecular formula:C4H7ClO3
- Molecular Weight:138.55

89-91-8
191 suppliers
$11.00/5g

13157-96-5
27 suppliers
inquiry
Yield:13157-96-5 100%
Reaction Conditions:
with iodine;acetyl chloride at 50; for 18 h;Product distribution / selectivity;
Steps:
11.a; 24.a
Example 11Synthesis of (Z)-3-[3'-(3-butyl-1-methylureido)biphenyl-4-yl]-2-methoxyacrylic acid a-Methyl Chloromethoxyacetate25 g (186 mmol) of methyl dimethoxyacetate, 26.5 mL (373 mmol) of acetyl chloride and 95 mg (0.4 mmol) of iodine are placed in a round-bottomed flask and heated at 50° C. for 18 hours. The reaction progress is monitored by NMR. The excess acetyl chloride is removed by evaporation under vacuum. 26 g (100%) of methyl chloromethoxyacetate are obtained in the form of a liquid colored brown by the residual iodine.; Example 24Synthesis of (Z)-3-[2-butoxy-3'-(1-methyl-3-pentylureido)biphenyl-4-yl]-2-methoxyacrylic acid a-Methyl Chloromethoxyacetate25 g (186 mmol) of commercial methyl dimethoxyacetate, 26.5 mL (373 mmol) of acetyl chloride and 95 mg (0.37 mmol) of iodine are placed in a round-bottomed flask and heated at 50° C. for 18 hours. The reaction progress is monitored by NMR. The excess acetyl chloride is removed by evaporation under vacuum P=150 mbar, at 35° C. (the final product has a fairly low boiling point). 27 g (100%) of methyl chloromethoxyacetate are obtained in the form of a liquid colored brown by the residual iodine.
References:
US2009/12129,2009,A1 Location in patent:Page/Page column 18; 25
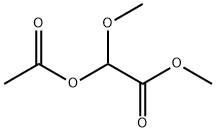
24607-11-2
0 suppliers
inquiry

13157-96-5
27 suppliers
inquiry
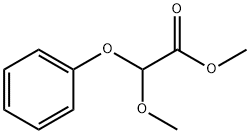
24607-12-3
0 suppliers
inquiry

13157-96-5
27 suppliers
inquiry
