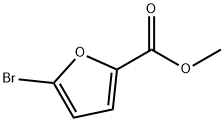
METHYL 5-BROMO-2-FUROATE synthesis
- Product Name:METHYL 5-BROMO-2-FUROATE
- CAS Number:2527-99-3
- Molecular formula:C6H5BrO3
- Molecular Weight:205.01

611-13-2
421 suppliers
$13.00/25g

2527-99-3
190 suppliers
$6.00/1g
Yield:2527-99-3 85%
Reaction Conditions:
with bromine at 50; for 0.5 h;Inert atmosphere;
Steps:
Methyl 5-bromo-2-furoate S6
Prepared according to the approach noted in Torii, S. et al., Anodic Reaction of 2-Furoic Acids. II. Electrolysis of Methyl 5-Acetyl-2-furoate and Its Homologous in Protic Solvents. Bull. Chem. Soc. Jpn. 1972, 45 (9), 2783-2787. DOI: 10.1246/bcsj.45.2783, the entire content of which is incorporated herein by reference. Bromine (6.07 g, 0.038 mol) was carefully added (dropwise over a period of 15 minutes) to a solution of methyl furoate (S5, 3.2 g, 0.025 mol) stirred at 50° C. under an argon atmosphere in a flame-dried round-bottom flask. The resulting dark orange/brownish solution was additionally stirred for another 15 minutes at 50° C. The reaction mixture was then poured into cold water (10 mL) and extracted with ethyl acetate (2×50 mL). The combined extracts were washed with water (1×10 mL) and brine (1×10 mL) prior to being dried with magnesium sulfate and concentrated. The final product was purified by flash chromatography (10:1, hexanes-ethyl acetate) to obtain 4.5 g of S6 in 85% yield. The spectral data was consistent with literature reports as noted in Torii above. Rf=0.17 (7:3, hexanes-ethyl acetate).
References:
University of Windsor US2021/40105, 2021, A1 Location in patent:Paragraph 0096

74-88-4
349 suppliers
$15.00/10g

2527-99-3
190 suppliers
$6.00/1g



