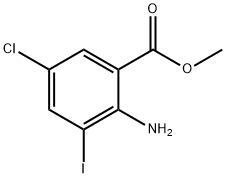
METHYL 2-AMINO-5-CHLORO-3-IODOBENZOATE synthesis
- Product Name:METHYL 2-AMINO-5-CHLORO-3-IODOBENZOATE
- CAS Number:289039-84-5
- Molecular formula:C8H7ClINO2
- Molecular Weight:311.5
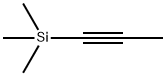
6224-91-5
247 suppliers
$12.00/1g

289039-84-5
47 suppliers
$60.00/100mg
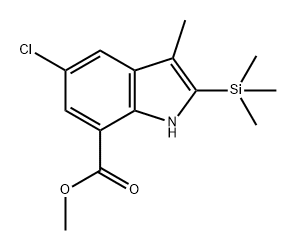
1262419-83-9
0 suppliers
inquiry
Yield:1262419-83-9 59%
Reaction Conditions:
with tetrabutyl-ammonium chloride;sodium carbonate;palladium diacetate in N,N-dimethyl-formamide at 100; for 15 h;Sealed tube;
Steps:
4.A
Step A: Tetrabutylammonium chloride (100 mg, 0.4 mmol),trimethyl(prop-l-ynyl)si lane (1.1 ml, 7.2 mmol), methyl 2-amino-5-chloro-3- iodobenzoate (1.0 g, 3.6 mmol), triphenylphosphine (47.2 mg, 0.1 mmol), palladium(II) acetate (20 mg, 0.1 mmol) and sodium carbonate (1.9 g, 18 mmol) in DMF were added to a sealed tube and the reaction was stirred at 100 0C for 15 h. After cooling to room temperature, the reaction mixture was diluted with ether and washed with saturated aqueous ammonium chloride and water. The organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude material was then purified by column chromatography (silica gel, 70:30 hexanes/ethyl acetate) to afford methyl 5- chloro-3-methyl-2-(trimethylsilyl)-lH-indole-7-carboxylate (620 mg, 59%) as a light yellow solid: 1H NMR (500 MHz, CDCI3) δ 9.50 (s, 1 H), 7.80 (d, J = 1.0 Hz, 1 H), 7.72 (d, J = 1.0 Hz, I H), 3.98 (s, 3H), 2.37 (s, 3H), 0.40 (t, 9H); MS (ESI+) m/z 296 (M+H).
References:
WO2011/8572,2011,A2 Location in patent:Page/Page column 55
