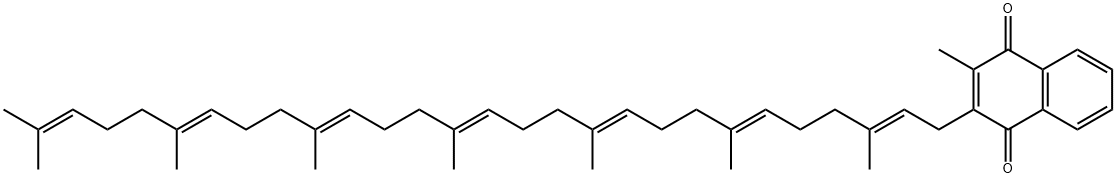
Menaquinone 7 synthesis
- Product Name:Menaquinone 7
- CAS Number:2124-57-4
- Molecular formula:C46H64O2
- Molecular Weight:649
![Naphthalene, 2-[(2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyl-2,6,10,14,18,22,26-octacosaheptaen-1-yl]-1,4-dimethoxy-3-methyl-](/CAS/20210305/GIF/1218784-62-3.gif)
1218784-62-3
0 suppliers
inquiry

2124-57-4
237 suppliers
$107.00/1mg
Yield:2124-57-4 80%
Reaction Conditions:
with ammonium cerium (IV) nitrate in water;acetonitrile at 20; for 4.08333 h;
Steps:
20 Example 20. Synthesis of Vitamin K2-7
Example 20. Synthesis of Vitamin K2-7 [0095] [0096] To a solution of 2-(3,7,11,15,19,23,27-Heptamethyl-octacosa-2,6,10,14,18,22,26-heptaenyl)-1,4-dimethoxy-3-methyl-naphthalene (Sch. 15. IV) (0.68, 0.001 mol) in acetonitrile (5 ml) was added an aqueous solution of ceric ammonium nitrate (1.1 g, 0.002 mol) portion wise over 5 min. The resultant reaction mixture was stirred at room temperature for 4 hours at which time the TLC indicated the completion of the reaction. All contents of the reaction flask were transferred to a separatory funnel containing chloroform (15 ml). The organic layer was washed with water (2 x 5 ml) and the aqueous layers were combined and extracted with chloroform (5 ml). The organic layers were combined, washed with brine (5 ml), dried over MgSO4, filtered and removed under vacuum to obtain vitamin K2-7 (0.52 g, 80% yield). 1HNMR(400 MHz, CDCl3) δ 8.05 (m, 2H), 7.64 (m, 2H), 5.05 (m, 7H), 3.35 (d, 2H, J = 7.0 Hz), 2.16 (s, 3H), 2.05 (m, 24H), 1.77 (s, 3H), 1.65 (s, 3H), 1.57 (s, 12 H), 1.53 (s, 6H).
References:
EP2868658,2015,A1 Location in patent:Paragraph 0095-0096
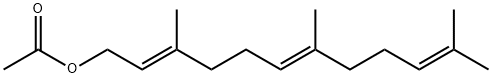
4128-17-0
24 suppliers
$125.00/5g

2124-57-4
237 suppliers
$107.00/1mg

93787-91-8
1 suppliers
inquiry

2124-57-4
237 suppliers
$107.00/1mg

104423-44-1
0 suppliers
inquiry

2124-57-4
237 suppliers
$107.00/1mg

68778-93-8
1 suppliers
inquiry

2124-57-4
237 suppliers
$107.00/1mg