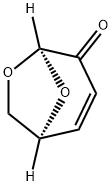
LEVOGLUCOSENONE synthesis
- Product Name:LEVOGLUCOSENONE
- CAS Number:37112-31-5
- Molecular formula:C6H6O3
- Molecular Weight:126.11
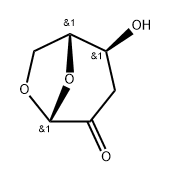
58238-45-2
0 suppliers
inquiry

37112-31-5
126 suppliers
$124.00/250mg
Yield:37112-31-5 76%
Reaction Conditions:
with acetic anhydride;acetic acid in dimethyl sulfoxide at 40; for 2 h;
Steps:
Methylsulfanylmethylation of hydroxy groups inalcohols 3, 4, 6, 7-10
General procedure: Method III (methylsulfanylmethylation with DMSO and Ac2O). A solution of alcohol 3, 4, 7, or 8 (5 mmol) in DMSO (10 ml) and 2 (10 ml) was stirred for 2 h at 40°. The reaction mixture was quenched by adding ice and saturated NaHCO3 solution until the evolution of gas ceased. The aqueous phase was extracted with EtOAc, the combined extracts were washed with NaHCO3 solution, water, dried over anhydrous MgSO4, evaporated, the residue was separated by chromatography on silica gel, using petroleum ether - EtOAc as eluent, gradient from 5:1 to 2:1. Method IV (methylsulfanylmethylation with DMSO,Ac2O, and AcOH). A solution of alcohol 3 or 4 (1.0 mmol)in a mixture of DMSO (1.5 ml), 2 (1.0 ml), and AcOH(0.4 ml) was stirred for 2 h at 40°. The reaction mixture was quenched by adding ice and saturated NaHCO3 solution until the evolution of gas ceased. The aqueous phase was extracted with EtOAc, the combined extracts were washed with NaHCO3 solution, water, dried over anhydrous MgSO4, evaporated, and the residue was separated by chromatography on silica gel, using petroleum ether - EtOAc as eluent, gradient from 5:1 to 2:1. Method V (methylsulfanylmethylation with Me2S,(PhCO2)2, MeCN). A solution of alcohol 3, 4, 6, 9, or 10(1.0 mmol) in anhydrous acetonitrile (4.0 ml) was cooled to 0° and treated by adding Me2S (0.58 ml, 8 mmol). After that, benzoyl peroxide (986 mg, 4 mmol) was added in 4 portions over 30 min intervals. The mixture was stirred for 2 h at 0°, diluted with Et2O, and then washed with saturated NaHCO3 solution. The aqueous phase was extracted with Et2O, dried over anhydrous MgSO4, evaporated, the residue was separated by chromatography on silica gel, using petroleum ether - EtOAc as eluent, gradient from 5:1 to 2:1
References:
Sharipov, Bulat T.;Davidova, Anna N.;Ryabova, Alena S.;Galimzyanova, Nailya F.;Valeev, Farid A. [Chemistry of Heterocyclic Compounds,2019,vol. 55,# 1,p. 31 - 37][Khim. Geterotsikl. Soedin.,2019,vol. 55,# 1,p. 31 - 37,7]
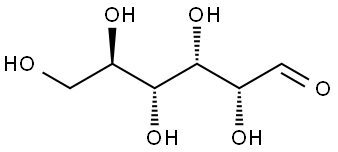
50-99-7
1008 suppliers
$6.00/25g

37112-31-5
126 suppliers
$124.00/250mg
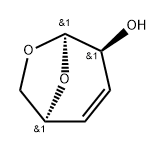
50705-28-7
2 suppliers
inquiry

37112-31-5
126 suppliers
$124.00/250mg

498-07-7
265 suppliers
$11.00/250mg

37112-31-5
126 suppliers
$124.00/250mg

498-07-7
265 suppliers
$11.00/250mg
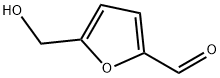
67-47-0
592 suppliers
$5.00/100mg

37112-31-5
126 suppliers
$124.00/250mg