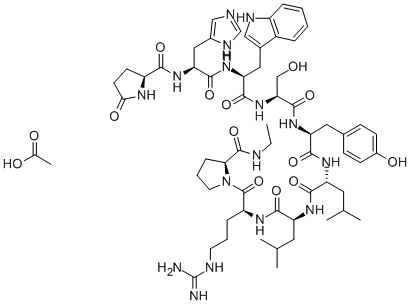
Leuprorelin synthesis
- Product Name:Leuprorelin
- CAS Number:53714-56-0
- Molecular formula:C59H84N16O12
- Molecular Weight:1209.4
(1) Fmoc-Pro-HMPB-AM resin is obtained from Fmoc-Pro-OH and HMPB-AM resin with a substitution degree of 0.2mmol/g~1.2mmol/g as starting materials;
(2) The Fmoc-Pro-HMPB-AM resin was coupled one by one by Fmoc/tBu solid phase method to connect amino acids with protective groups in sequence, and the side chain fully protected leuprolide precursor peptide-HMPB-AM was synthesized Resin;
(3) Cut the side chain fully protected leuprolide precursor peptide-HMPB-AM resin to obtain the side chain fully protected leuprolide precursor peptide;
(4) Fully protected side chain leuprolide precursor peptide undergoes ethylamination to obtain side chain fully protected leuprolide;
(5) Leuprolide is fully protected on the side chain by removing the side chain protecting group to obtain the crude leuprolide peptide;
(6) The crude leuprorelin peptide is separated and purified by a high-pressure liquid phase column and lyophilized to obtain the leuprolide refined peptide.
914200-76-3
0 suppliers
inquiry

53714-56-0
271 suppliers
$55.00/1mg
Yield:-
Reaction Conditions:
with chlorotriisopropylsilane;ethane-1,2-dithiol;trifluoroacetic acid at 20; for 2 h;
Steps:
3
The protecting groups from pGlu-His(Trt)-Trp-Ser(tBu)-Tyr(tBu)-D-Leu-Leu-Arg(Pbf)-Pro-NHEt (SEQ. ID. NO. 1) (about 1000 g obtained in Example 2) were removed using a 95% TFA, 2.5% TIS, 2.5% EDT solution for 2 hours at room temperature. The product was precipitated by the addition of 10 volumes of MTBE, filtered, and dried in vacuum to obtain 680 g product.
References:
Tovi, Avi;Eidelman, Chaim;Shushan, Shimon;Elster, Shai;Alon, Hagi;Ivchenko, Alexander;Butilca, Gabriel-Marcus;Zaovi, Gil;Alterman, Eleonora;Bar-Oz, Leah;Gadi, Tehila US2006/276626, 2006, A1 Location in patent:Page/Page column 13