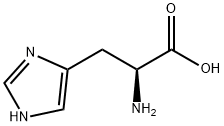
L-Histidine synthesis
- Product Name:L-Histidine
- CAS Number:71-00-1
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
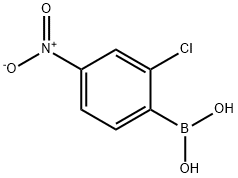
1436612-57-5
17 suppliers
$315.00/250mg

71-00-1
737 suppliers
$7.00/5g
Yield:71-00-1 84.5%
Reaction Conditions:
with palladium in methanol;cyclohexane; for 120 h;Reflux;
Steps:
L-Histidine dihydrochloride (1).
tert-Butyl-3-(1-benzyl-1H-imidazol-5-yl)-2-(diphenylmethyleneamino)propanoate (11, 400 mg, 0.86 mmol) is dissolved in THF (10 mL) and then stirred with 10% citric acid solution (5 mL) overnight. TLC shows complete disappearance of the starting material. The mixture is extracted twice with ether (20 mL). The water layer is then brought to pH 12 with a K2CO3 solution and extracted with ethyl acetate (10 mL). The combined organic layers are dried over MgSO4 and concentrated in vacuo to give brown crystals (250 mg, 0.828 mmol, 96.3%). This compound is then treated with 1 N HCl (5 mL), and the mixture is refluxed overnight. The reaction is followed byTLC until the starting material has disappeared (dichloromethane/ethanol = 99/1). The mixture is allowed to reach room temperature, diluted with a small amount of dichloromethane, filtered and concentrated in vacuo. The product is a yellow oil (230 mg, 0.709 mmol, 85.7%). The latter is dissolved in a mixture of cyclohexene/methanol (1/1). Palladium black (100 mg) is added to the mixture. The mixture is refluxed until the conversion is complete, which takes 5 days. On the second day a freshly prepared Pd pellet [6] (50 mg in 10 mL of the same solvent mixture) is added to the mixture. The suspension is cooled down to room temperature and filtered over Celite. The solvent is evaporated in vacuo; the compound is redissolved in methanol and then concentrated to yield a yellow solid (165 mg, 0.727 mmol, 84.5%). 1H-NMR (400 MHz, D2O/DCl, δ in ppm): 3.46 (1H, H3a), 3.62(1H, H3b), 4.42 (1H, H2), 7.49 (1H, H5'), 8.74 (1H, H2'); 13C-NMR (100 MHz, D2O/DCl, δ in ppm):27.9 (C3), 54.7 (C2), 118.5 (C5'), 130.0 (C4'), 135.8 (C2'), 174.0 (C1).
References:
Talab, Sarra;Taha, Kamal Khalifa;Lugtenburg, Johan [Molecules,2014,vol. 19,# 1,p. 1023 - 1033]

305-84-0
704 suppliers
$10.00/1g

71-00-1
737 suppliers
$7.00/5g

2002-22-4
63 suppliers
$87.35/100MG

71-00-1
737 suppliers
$7.00/5g
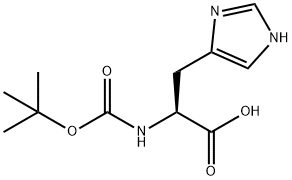
17791-52-5
287 suppliers
$5.00/1g

71-00-1
737 suppliers
$7.00/5g
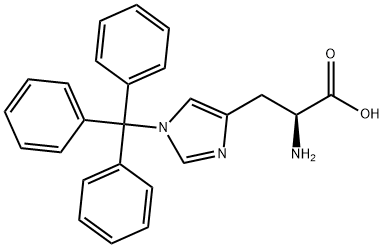
35146-32-8
162 suppliers
$23.00/1g

71-00-1
737 suppliers
$7.00/5g