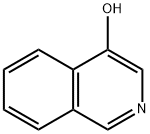
Isoquinolin-4-ol synthesis
- Product Name:Isoquinolin-4-ol
- CAS Number:3336-49-0
- Molecular formula:C9H7NO
- Molecular Weight:145.16
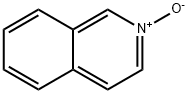
1532-72-5
165 suppliers
$15.00/1g

3336-49-0
92 suppliers
$32.00/250 mg
Yield: 10.6 g (48.4%)
Reaction Conditions:
with sulfuric acid;sodium carbonate;acetic acid in chloroform;water
Steps:
4.b b
b 4-Hydroxyisoquinoline (cf. E. Ochai, M. Tkebara, Pharm. Bull. 3 (1956), 454) 21.8 g (150 mmol) of isoquinoline N-oxide are dissolved in 400 ml of chloroform, thoroughly dried over magnesium sulfate and filtered. To this solution are added 31.2 g (164 mmol) of freshly recrystallized p-toluenesulfonic chloride, which dissolves with the evolution of heat. A solution of 20 g of sodium carbonate in 450 ml of water is added with vigorous stirring. This solution is stirred at room temperature for 15 hours. The organic phase is separated off, dried and the solvent is distilled off. 440 ml of 5M sulfuric acid are added to the red, oily residue obtained, and the entire mixture is heated to boiling for 24 hours. The cooled solution is neutralized with solid sodium carbonate, and the resulting light brown precipitate is filtered off. Yield 10.6 g (48.4%)--melting point 207° C. (from acetone/acetic acid (6:1), dec.)
References:
Riedel-De-Haen US5266700, 1993, A
1532-97-4
350 suppliers
$14.00/10g

3336-49-0
92 suppliers
$32.00/250 mg

1532-72-5
165 suppliers
$15.00/1g

3336-49-0
92 suppliers
$32.00/250 mg
1532-91-8
82 suppliers
$41.00/1g

3336-49-0
92 suppliers
$32.00/250 mg
55270-33-2
75 suppliers
$74.00/250mg

3336-49-0
92 suppliers
$32.00/250 mg
