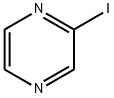
Iodopyrazine synthesis
- Product Name:Iodopyrazine
- CAS Number:32111-21-0
- Molecular formula:C4H3IN2
- Molecular Weight:205.98
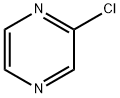
14508-49-7
368 suppliers
$14.00/25g

32111-21-0
163 suppliers
$7.00/1g
Yield:32111-21-0 60%
Reaction Conditions:
Stage #1:2-chloropyrazin with acetic acid;sodium iodide;sulfuric acid in acetonitrile for 4.5 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water
Steps:
1
A reaction mixture of chloropyrazine (7.5 ml, 83 mmol), NaI (30.3 g, 202 15 mmol), HOAc (9.6 ml, 168 mmol) and H2SO4 (0.5 ml) in MeCN (105 ml) was heated at reflux for 4.5 hours. The solvent was removed and water (120 ml) was added. After the solution was basified with saturated NaHCO3, it was extracted with dichloromethane (DCM) (2 x 125 ml). The DCM layers were combined, washed with saturated Na2S2O3, brine and dried. The removal of solvent gave crude iodopyrazine as an oil (12.33 g, 20 71%). Analysis by 1H NMR showed there was less than about 10 mol% of chloropyrazine in the oil. Another batch of chloropyrazine (50 g, 437 mmol) was also converted into crude iodopyrazine (about 65 g) by the same procedure. These two batches of crude iodopyrazine were combined and distillation of the crude iodopyrazine under reduced pressure (about 0.75 torr, bp 47°C) gave pure compound 64 g (60%). 251H-NMR (CDCl3, 300MHz) 8.40 (dd, /=1.8, 2.4Hz, IH), 8.51 (d, /=2.4Hz,lH), 8.87 (d, /=1.5Hz,lH).
References:
INTERNATIONAL PARTNERSHIP FOR MICROBICIDES WO2009/114313, 2009, A2 Location in patent:Page/Page column 15
290-37-9
305 suppliers
$5.00/5g

32111-21-0
163 suppliers
$7.00/1g

290-37-9
305 suppliers
$5.00/5g

10199-00-5
76 suppliers
$60.00/100mg

32111-21-0
163 suppliers
$7.00/1g
5049-61-6
432 suppliers
$8.00/10g

32111-21-0
163 suppliers
$7.00/1g

290-37-9
305 suppliers
$5.00/5g
1093418-77-9
25 suppliers
$150.50/50 mg

32111-21-0
163 suppliers
$7.00/1g
