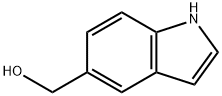
INDOLE-5-METHANOL synthesis
- Product Name:INDOLE-5-METHANOL
- CAS Number:1075-25-8
- Molecular formula:C9H9NO
- Molecular Weight:147.17
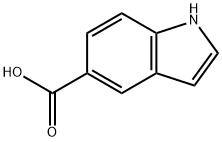
1670-81-1
340 suppliers
$6.00/250mg

1075-25-8
120 suppliers
$73.00/250mg
Yield: 93%
Reaction Conditions:
Stage #1:1H-Indole-5-carboxylic acid with lithium aluminium tetrahydride in tetrahydrofuran at 20;Reflux;
Stage #2: with water;sodium hydroxide in tetrahydrofuran
Steps:
37.1
To a mechanically stirred solution of indole-5-carboxylic acid (26.3g, 163mmol) in tetrahydrofuran (60OmL), was added a solution of lithum aluminium hydride 1.0M in tetrahydrofuran (20OmL, 200mmol) drop wise at ambient temperature. A solution of lithium aluminium hydride 2.0M in tetrahydrofuran (22mL, 44mmol) was added drop wise at ambient temperature, and then the reaction mixture was carefully heated up to reflux and held there for 1 hour. The reaction mixture was cooled to ambient temperature and then water (13mL) was added drop wise, followed by an aqueous 10% sodium hydroxide solution (13mL), and water (2OmL). The suspension was stirred at ambient temperature for 30 minutes, filtered through a bed of celite and the filter cake washed with ethyl acetate (2x200mL). The filtrate and the washings were combined and the organics were separated, dried (Na2SO4) and concentrated in vacuo, to give a dark red oil. The resultant crude product was suspended in hexane (20OmL)1 and ethyl acetate (1OmL) was added. This mixture was stirred at ambient temperature for 18 hours, and the solids were separated via filtration and washed with hexane to afford the title compound as a light brown solid, 22.25g, 93%.
References:
VERNALIS (R & D) LTD WO2009/93012, 2009, A1 Location in patent:Page/Page column 26; 65
1196-69-6
308 suppliers
$9.00/1g

1075-25-8
120 suppliers
$73.00/250mg
1011-65-0
279 suppliers
$6.00/250mg

1075-25-8
120 suppliers
$73.00/250mg
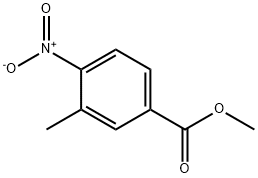
24078-21-5
246 suppliers
$6.00/5g

1075-25-8
120 suppliers
$73.00/250mg
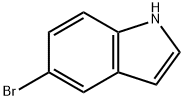
10075-50-0
688 suppliers
$5.00/10g

1075-25-8
120 suppliers
$73.00/250mg