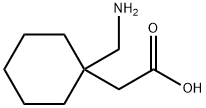
Gabapentin synthesis
- Product Name:Gabapentin
- CAS Number:60142-96-3
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
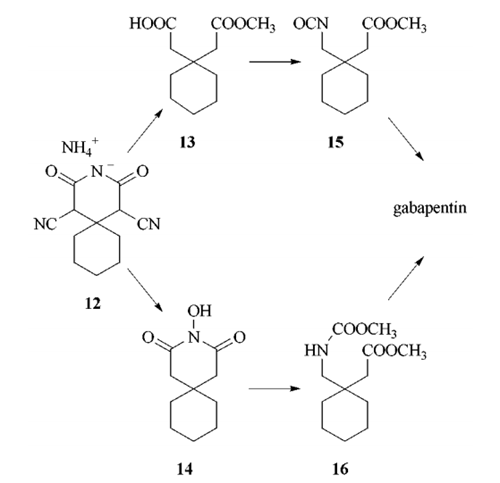
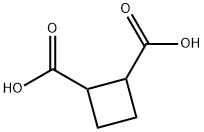
3396-14-3
70 suppliers
$183.00/250mg

60142-96-3
767 suppliers
$9.34/5g
Yield:60142-96-3 98%
Reaction Conditions:
in tetrahydrofuran;water;
Steps:
39.39 Synthesis of Compound 1
Example 39 Synthesis of Compound 1 Lithium aluminum hydride (69.4 mL of a 1 M solution in ether, 69.4 mmol) was added dropwise to a stirring solution of cis-cyclobutane-1,2-dicarboxylic acid (5 g, 34.7 mmol) in THF (60 mL) at 0° C. under argon. The mixture was allowed to warm to room temperature and stirred for 16 hours. The mixture was cooled to 0° C. and quenched by careful addition of water (2.7 mL), sodium hydroxide solution (2.7 mL of a 15% w/v solution), and water (8.1 mL). The mixture was stirred for 15 minutes, and the precipitate was removed by filtration. The solvent was evaporated under reduced pressure to give the alcohol 1 as a colorless oil (4.0 g, 98%); δH (400 MHz; CDCl3): 3.85 (2H, m), 3.6 (2H, m), 3.2 (2H, s), 2.7 (2H, m), 2 (2H, m); 1.55 (2H, m); δC (400 MHz; CDCl3): 63.15, 37.83, 20.40.
References:
US2002/72533,2002,A1
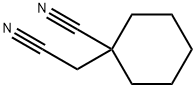
4172-99-0
62 suppliers
inquiry

60142-96-3
767 suppliers
$9.34/5g

133481-09-1
107 suppliers
$151.00/y0001348

60142-96-3
767 suppliers
$9.34/5g
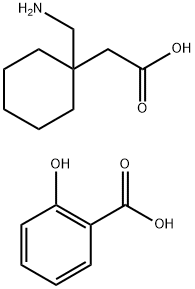
756486-04-1
3 suppliers
inquiry

60142-96-3
767 suppliers
$9.34/5g
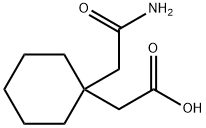
99189-60-3
368 suppliers
$20.00/1g

60142-96-3
767 suppliers
$9.34/5g