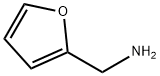
Furfurylamine synthesis
- Product Name:Furfurylamine
- CAS Number:617-89-0
- Molecular formula:C5H7NO
- Molecular Weight:97.12
SIMPLE, NOVEL SYNTHESIS OF FURFURYLAMINE FROM FURFURAL BY ONE-POT REDUCTIVE AMINATION IN WATER USING ZINC METAL

98-01-1
483 suppliers
$22.00/25g

617-89-0
272 suppliers
$16.00/25g
Yield:617-89-0 96%
Reaction Conditions:
with ammonium hydroxide;hydrogen in diethyl ether at 50; under 7500.75 Torr; for 36 h;
Steps:
3
Add 1 mmol of furfural, a ruthenium-based catalyst with a molar amount of furfural of 20 mol %, ammonia water (25 wt %) with a molar ratio of furfural of 2, and 5 mL of ether into a 15 mL reaction kettle, close the kettle, replace the air in the kettle with hydrogen 5 times, and fill it with 1.0MPa hydrogen, heated to 50°C, and reacted at this temperature for 36h.After the reaction, according to the method described in Example 1, cooling and sampling analysis, the conversion rate of furfural was 99%, and the GC yield of furfurylamine was 97%.The isolated yield was 96%.
References:
CN113717054,2021,A Location in patent:Paragraph 0083; 0085

98-01-1
483 suppliers
$22.00/25g
4795-29-3
176 suppliers
$30.05/25ML

617-89-0
272 suppliers
$16.00/25g

98-01-1
483 suppliers
$22.00/25g

18240-50-1
18 suppliers
$60.00/50mg

617-89-0
272 suppliers
$16.00/25g

617-90-3
151 suppliers
$11.00/1g

617-89-0
272 suppliers
$16.00/25g

98-01-1
483 suppliers
$22.00/25g

19377-82-3
7 suppliers
inquiry

617-89-0
272 suppliers
$16.00/25g
550-23-2
4 suppliers
inquiry