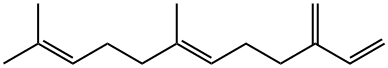
(E)-BETA-FARNESENE synthesis
- Product Name:(E)-BETA-FARNESENE
- CAS Number:18794-84-8
- Molecular formula:C15H24
- Molecular Weight:204.35
Yield:-
Reaction Conditions:
with dmap;tributyl-amine;palladium diacetate;acetic anhydride;bis[2-(diphenylphosphino)phenyl] ether at 115; for 4 h;Inert atmosphere;Large scale;Reagent/catalyst;Time;
Steps:
1; 2; 3; 4 General conditions of the Pd-catalyzed elimination via in-situ acetates
Acetic anhydride (2.6 kg, 25.3 mol) is mixed with E-Nerolidol (1.1 kg, 5 mol) under nitrogen and stirring, followed by tributylamine (1.9 kg, 10.1 mol), DMAP (32 g), DPEphos (1.55 g, 2.9 mmol) and Pd(OAc)2 (0.3 g, 1.25 mmol, 0.025 mol%). The mixture is heated to 115 °C, stirred at this temperature for 4 h, then cooled to 70 °C. Vacuum (25 - 55 mbar) is applied and excess acetic anhydride distilled at 66 °C head temperature giving 1.9 kg of an acetic anhydride / tributylamine mixture (ratio 83:17). The distillation residue is cooled to 15 °C and a mixture of 32% HCI (1.43 kg) and H20 (1 kg) is added at 15 - 20 °C under stirring. The 2- phase mixture is heated to 40 °C and extracted with terf-butyl methyl ether (2 x 2 I). The combined organic layers are washed with 2 kg of water, 0.5 kg brine and saturated NaHC03 (2.5 I). After partial evaporation of tert- butyl methyl ether, toluene (1 I) is added. Evaporation under reduced pressure gives 999 g of crude Farnesene which is wipe-film-distilled at 105 °C / 0.3 mbar giving 945 g of 6-E-Farnesene (93% yield) as a 1 :2 mixture of a- and /2-isomers (purity 90%). The analytical data are identical to the ones described for 6-E-Farnesene, e.g. by E.J. Corey et al. J. Am. Chem. Soc. 140, 16909 - 16913 (2018).
References:
WO2021/255052,2021,A1 Location in patent:Page/Page column 26-28
![Benzene, [[(1Z,5E)-2-ethenyl-6,10-dimethyl-1,5,9-undecatrien-1-yl]dimethylsilyl]-](/CAS/20210305/GIF/87513-97-1.gif)
87513-97-1
0 suppliers
inquiry

18794-84-8
131 suppliers
$28.00/1G
![Benzene, [[(6E)-7,11-dimethyl-3-methylene-6,10-dodecadien-1-yl]sulfinyl]-](/CAS/20210305/GIF/72445-10-4.gif)
72445-10-4
0 suppliers
inquiry

18794-84-8
131 suppliers
$28.00/1G

40716-66-3
189 suppliers
$6.00/5g

18794-84-8
131 suppliers
$28.00/1G
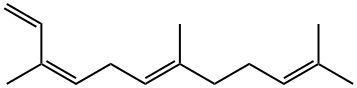
26560-14-5
5 suppliers
inquiry
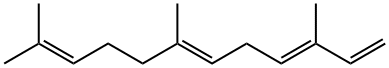
502-61-4
82 suppliers
inquiry
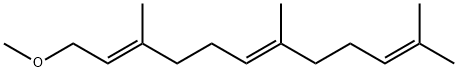
540-06-7
2 suppliers
inquiry

18794-84-8
131 suppliers
$28.00/1G