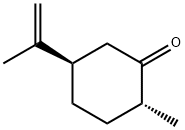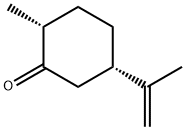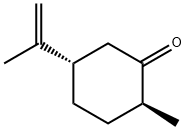
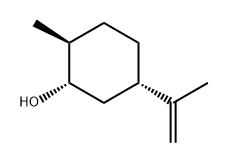
(-)-DIHYDROCRAVONE synthesis
- Product Name:(-)-DIHYDROCRAVONE
- CAS Number:619-02-3
- Molecular formula:C10H16O
- Molecular Weight:152.23
Yield:89 % de
Reaction Conditions:
with Gluconobacter oxydans old yellow enzyme family protein Gox0502 (a.a 1–315), recombinant, W100Y mutant;NADPH in aq. phosphate buffer;ethanol at 30; pH=7; for 1 h;Enzymatic reaction;Overall yield = 36.2 %; diastereoselective reaction;Reagent/catalyst;
Steps:
Biocatalysis assay and data analysis.
General procedure: Biotransformationswere typically performed in a standard reactionsystem containing 30 μg/mL enzyme, 2 mM substrate,2.1 mM NADPH, 100 mM PBS buffer pH 7.0, and10% (v/v) ethanol to a final volume of 0.5 mL. Themixtures were incubated in an orbital shaker at150 rpm at 30 °C for 1 h. Reactions were terminatedby extraction through chloroform (0.5 mL) containing0.15% (v/v) 2-octanone (as internal standard). Aftercentrifugation, the lower organic phase samples weretaken for GC (Gas chromatography) analysis.Concentrations and diastereomeric excesses (de) weredetermined by a GC-6890 N equipped with a30 m × 0.25 mm DB-5 column (Agilent Technologies).The injection volume was 1 μL with a split ratio of 10: 1.The following program was applied for GC analysis:40 °C for 3 min; 10 °C/min increase to 220 °C and maintainedat 220 °C for 3 min. For (S)-carvone’s catalysisexperiment, the corresponding retention times were asfollows: (S)-carvone 14.28 min, (1R, 4S)-dihydrocarvone13.60 min, and (1S, 4S)-dihydrocarvone 13.72 min. For(R)-carvone’s catalysis experiment, the correspondingretention times were as follows: (R)-carvone 14.28 min,(1R, 4R)-dihydrocarvone 13.72 min, and (1S, 4R)-dihydrocarvone13.60 min. The concentrations of the reactionproducts were calculated based on absorption. 2-Octanonewas used as an internal standard, which had beensubjected to chloroform during the extraction step.
References:
Yin, Bo;Deng, Jian;Lim, Lirong;Yuan, Y. Adam;Wei, Dongzhi [Bioscience, Biotechnology and Biochemistry,2015,vol. 79,# 3,p. 410 - 421]

6485-40-1
328 suppliers
$12.00/25g

619-02-3
3 suppliers
inquiry

1629-58-9
130 suppliers
$56.79/5g

6485-40-1
328 suppliers
$12.00/25g

619-02-3
3 suppliers
inquiry
547-26-2
2 suppliers
inquiry
22567-21-1
22 suppliers
$114.00/1mL

619-02-3
3 suppliers
inquiry