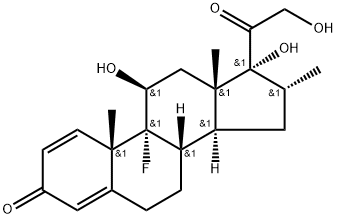
Dexamethasone synthesis
- Product Name:Dexamethasone
- CAS Number:50-02-2
- Molecular formula:C22H29FO5
- Molecular Weight:392.47
Dexamethasone is synthesized in a multistage process from 3α-acetoxy-16-pregnen- 11,20-dione, which is reacted with methylmagnesium bromide in the presence of lithium bromide to give 3α-hydroxy-16α-methylpregnan-11,20-dione (27.1.39), after which a 17α- hydroxyl group is added. This is done by a reaction with acetic anhydride in the presence of p-toluenesulfonic acid, forming the 3-acetoxy-17-enolacetate 27.1.40, which is epoxidized by perbenzoic acid 27.1.41, and the product is hydrolyzed by an alkali to give an oxyketone 27.1.42. Addition of another hydroxyl group at C21 is accomplished by subsequent bromination of a methyl group with molecular bromine, replacing the bromine atom with iodine, and reacting iodide with potassium acetate, which forms the corresponding acetoxyketone 27.1.43. The hydroxyl group at C3 is oxidized to a carbonyl by chromium(VI) oxide in pyridine, giving the 3,11,20-triketone 27.1.44, which again undergoes bromination by molecular bromine, but at position C4. Dehydrogenation of this compound is accomplished using semicarbazide, which results in the formation of an unsaturated triketone 27.1.45. In order to avoid formation of semicarbazones at the keto-groups at C3 and C20, the final product is treated with pyruvic acid. Semicarbazones are then specially formed at the keto-groups of C3 and C20, and the keto-group at C11 that does not take part in semicarbazone formation is reduced to hydroxyl group using sodium borohydride. After removing the protective semicarbzone groups, 21-O-acetoxy-16β-methylhydrocortisone (27.1.46) is formed. This is reacted with potassium acetate and transformed to the epoxide 27.1.49. Reacting this with hydrofluoric acid results in an opening of the epoxide ring, during which the fluorohydrin 27.1.50 is formed. Finally, microbiological dehydrogenation of this compound at C1–C2 and simultaneous deacetylation gives dexamethasone (27.1.51).

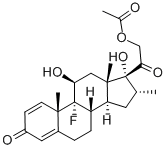
1177-87-3
478 suppliers
$29.00/100mg

50-02-2
713 suppliers
$20.00/1G
Yield:-
Reaction Conditions:
with methanol;sodium carbonate at 20; for 0.166667 h;Temperature;
Steps:
1.3
3), base-cataylzed hydrolysis: add the obtained dexamethasone acetate in step 2 to Na2CO3 and methanol. Reaction temperature is 20degC. After reacting for 10min, obtain dexamethasone. Dexamethasone acetate, Na2CO3, methanol has a molar ratio of 1:4:214;
References:
CN105348358,2016,A Location in patent:Paragraph 0014
![Pregna-1,4-dien-3-one, 9,11-epoxy-16-methyl-17,20:20,21-bis[methylenebis(oxy)]-, (9β,11β,16α)- (9CI)](/CAS/20210305/GIF/1966-25-2.gif)
1966-25-2
0 suppliers
inquiry

50-02-2
713 suppliers
$20.00/1G
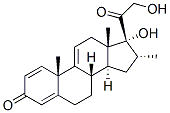
13209-41-1
83 suppliers
$110.00/5mg

50-02-2
713 suppliers
$20.00/1G
![Pregna-1,4,9(11)-trien-3-one, 16-methyl-17,20:20,21-bis[methylenebis(oxy)]-, (16α)- (9CI)](/CAS/20210305/GIF/14518-56-0.gif)
14518-56-0
0 suppliers
inquiry

50-02-2
713 suppliers
$20.00/1G
![Pregna-1,4-dien-3-one, 9-bromo-11-hydroxy-16-methyl-17,20:20,21-bis[methylenebis(oxy)]-, (11β,16α)- (9CI)](/CAS/20210305/GIF/88509-22-2.gif)
88509-22-2
0 suppliers
inquiry

50-02-2
713 suppliers
$20.00/1G