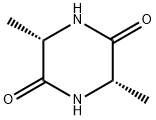
CYCLO(-ALA-ALA) synthesis
- Product Name:CYCLO(-ALA-ALA)
- CAS Number:5845-61-4
- Molecular formula:C6H10N2O2
- Molecular Weight:142.16

15761-38-3
521 suppliers
$5.00/10g

5845-61-4
49 suppliers
$45.00/25mg
Yield:-
Reaction Conditions:
Stage #1:L-N-Boc-Ala with benzotriazol-1-ol in dichloromethane;N,N-dimethyl-formamide at 0;
Stage #2: with dmap;N-ethyl-N,N-diisopropylamine;diisopropyl-carbodiimide in dichloromethane;N,N-dimethyl-formamide at 20; for 3 h;
Stage #3:L-N-Boc-AlaFurther stages;
Steps:
4.2. Preparation of CDPs (3-35)
General procedure: 4.2.1. Coupling of the first N-Boc protected α-aminoacid on oxime resinA desired quantity of oxime resin (1.12 mmol/g) wasadded to a peptide synthesis vessel. The resin was treatedthree times with CH2Cl2. Amino acid (3.0 equiv) and HOBt(3.0 equiv) were dissolved in DMF in a 100-mL flask andthe mixture was stirred for few minutes at 0 °C. DIC (3.0equiv), DIEA (3.0 equiv) and DMAP (0.1 equiv) wereadded and the mixture was introduced into the peptidesynthesis vessel and stirred mechanically for 3 h. Themixture was filtered under vacuum and the resin waswashed (DMF (3 × 100 mL), MeOH (3 × 100 mL), DMF(3 × 100 mL), MeOH (3 × 100 mL)) and dried under reducedpressure.4.2.2. Acetylation of unreacted sites on oxime resinThe resin was treated three times with DMF (3 × 50 mL).A solution of 50% v/v DMF/acetic anhydride (80 mL) andDIEA (1 mL) were added to the peptide synthesis vesseland shaken for 1 h. Then, the mixture was filtered undervacuum and the resin was washed (DMF (3 × 100 mL),MeOH (3 × 100 mL), DMF (3 × 100 mL), MeOH (3 × 100 mL))and dried under reduced pressure.4.2.3. Removal of the N-Boc protecting groupThe resin was treated three times with CH2Cl2 (100 mL). A50% v/v solution of TFA in CH2Cl2 was added to the peptidesynthesis vessel and shaken for 30 min. Then, the mixturewas filtered under vacuum and the resin was washed withDMF (3 × 100 mL), MeOH (3 × 100 mL), DMF (3 × 100 mL),MeOH (3 × 100 mL) and with a solution of 10% v/v DIEAin CH2Cl2 (100 mL).4.2.4. Coupling of the second N-Boc protectedα-amino acidThe amino acid (3.0 equiv) was dissolved in DMF in a100 mL flask. The solution was cooled to 0 °C, then HBTU(3.0 equiv) and HOBt (3.0 equiv) were added. The mixturewas poured into the peptide synthesis vessel in which theresin has been previously treated with CH2Cl2. DIEA (6.0equiv) was also added to the vessel and the mixture wasshaken for 3 h. After filtration under vacuum, the resinwas washed (DMF (3 × 100 mL), MeOH (3 × 100 mL), DMF(3 × 100 mL) and MeOH (3 × 100 mL)) and dried underreduce pressure. The Kaiser ninhydrin test was performedto monitor the efficiency of the coupling, and the couplingprocedure was repeated if needed.4.2.5. Cyclisation/cleavage from the resinFirst, N-Boc group was removed using procedure describedin (Section 4.2.3), but without the 10% v/v DIEA/CH2Cl2 washing step. After drying, CH2Cl2 and DIEA (2.5 equiv)were added to the peptide synthesis vessel and the mixturewas shaken for 2 min. Acetic acid (5.0 equiv) was thenadded and the content was shaken for 24 h. Then the filtratewas collected and the resin was rinsed several timeswith CH2Cl2 and MeOH. All the filtrates were combinedand evaporated, and the resulting solid was dissolved inCH2Cl2. Amberlite IR-120 was introduced to the solution toremove remaining traces of DIEA. The mixture was stirredfor a few minutes and filtered. The filtrate was evaporatedto give compounds 3 to 35. Trituration in a minimum ofcold ether was performed and led to desired compoundswith satisfying purity.
References:
Bérubé, Christopher;Barbeau, Xavier;Cardinal, Sébastien;Boudreault, Pierre-Luc;Bouchard, Corinne;Delcey, Nicolas;Lagüe, Patrick;Voyer, Normand [Supramolecular Chemistry,2017,vol. 29,# 5,p. 330 - 349]
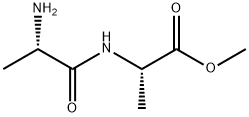
24326-09-8
2 suppliers
inquiry

5845-61-4
49 suppliers
$45.00/25mg

1948-31-8
149 suppliers
$13.00/100mg

5845-61-4
49 suppliers
$45.00/25mg
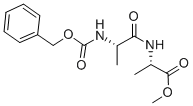
2483-51-4
45 suppliers
$17.00/1g

5845-61-4
49 suppliers
$45.00/25mg
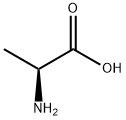
56-41-7
793 suppliers
$5.00/5g

5845-61-4
49 suppliers
$45.00/25mg