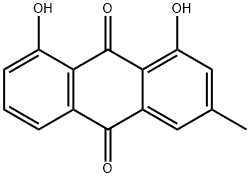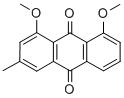
chrysophanol dimethyl ether synthesis
- Product Name:chrysophanol dimethyl ether
- CAS Number:71013-35-9
- Molecular formula:C17H14O4
- Molecular Weight:282.294
Yield:71013-35-9 89%
Reaction Conditions:
with potassium carbonate in acetone; for 12 h;Reflux;
Steps:
4.2. General method for the preparation of ether derivatives ofnepodin and chrysophanol
General procedure: A conventional method of O-alkylation using potassium carbonate (K2CO3) as a base in aprotic solvent was used. To a stirred solution of 1 (0.69 mmol) containing potassium carbonate (1.17 mmol) in dry acetone (8e10 mL), an appropriate alkylating agent (1.04 mmol) was added dropwise and resulting reaction mixture was refluxed for 15-24 h. Progress of reaction was monitored by TLC. On completion, the reaction was quenched with water and aqueous portion was extracted with EtOAc (3 30 mL). Organic layer was passed over anhydrous Na2SO4 and solvent was removed under vacuum. The crude product was purified by CC (hexane/ethylacetate 98:2) to give desired 1- and 8-O-alkylated nepodin derivatives. Similar procedure was followed for the preparation of chrysophanol derivatives using 4 equiv of both potassium carbonate and alkylating agents.
References:
Grover, Jagdeep;Kumar, Vivek;Singh, Vikram;Bairwa, Khemraj;Sobhia, M. Elizabeth;Jachak, Sanjay M. [European Journal of Medicinal Chemistry,2014,vol. 80,p. 47 - 56]
14963-96-3
34 suppliers
$30.00/100mg

100-84-5
255 suppliers
$9.00/25g

71013-35-9
3 suppliers
inquiry
121275-42-1
0 suppliers
inquiry

71013-35-9
3 suppliers
inquiry
61836-33-7
0 suppliers
inquiry
![Silane, [[(1E)-1-methoxy-3-methyl-1,3-butadien-1-yl]oxy]trimethyl-](/CAS/20210305/GIF/76927-59-8.gif)
76927-59-8
0 suppliers
inquiry
3300-25-2
47 suppliers
inquiry

71013-35-9
3 suppliers
inquiry