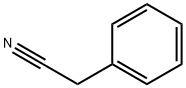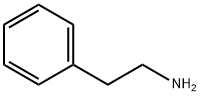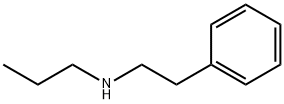
CHEMBRDG-BB 6382736 synthesis
- Product Name:CHEMBRDG-BB 6382736
- CAS Number:27906-91-8
- Molecular formula:C11H17N
- Molecular Weight:163.26
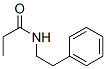
6283-04-1
1 suppliers
inquiry

27906-91-8
11 suppliers
$46.00/1g
Yield:27906-91-8 91%
Reaction Conditions:
with C20H25Cl2CoN3;sodium triethylborohydride in 1,2-dimethoxyethane at 100; for 6 h;Inert atmosphere;
Steps:
19 Example 19: Reduction of N-(2-phenylethyl)propionamide to N-propylphenethylamine:
Under an inert atmosphere, the substrate N-(2-phenylethyl)propionamide (177 mg, 1 mmol), polymethylhydrosiloxane (668 μL, 3 mmol), and Co-2 catalyst (9.0 mg, 0.02 mmol), sodium triethylborohydride (40 μL, 0.04 mmol), and ethylene glycol dimethyl ether (2 mL), and the resulting mixture was stirred well.The reaction was carried out in an oil bath at 100°C for 6 h, the reaction system was cooled to room temperature, ethyl acetate was added to dilute and quench, and concentrated, and the crude product was obtained by flash silica gel column chromatography to obtain 148 mg of light yellow oily liquid, yield: 91%.
References:
CN114031477,2022,A Location in patent:Paragraph 0087-0089
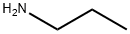
107-10-8
362 suppliers
$10.00/20mg
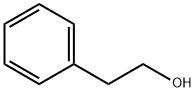
60-12-8
563 suppliers
$6.00/25g

27906-91-8
11 suppliers
$46.00/1g

100-42-5
636 suppliers
$20.00/25mL
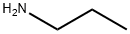
107-10-8
362 suppliers
$10.00/20mg

27906-91-8
11 suppliers
$46.00/1g