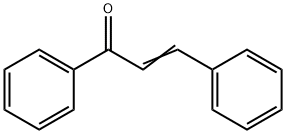
Chalcone synthesis
- Product Name:Chalcone
- CAS Number:94-41-7
- Molecular formula:C15H12O
- Molecular Weight:208.26
Chalcones can be prepared by an aldol condensation between a benzaldehyde and an acetophenone in the presence of sodium hydroxide as a catalyst.
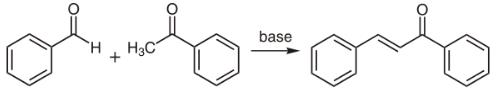
This reaction has been found to work without any solvent at all - a solid-state reaction. The reaction between substituted benzaldehydes and acetophenones has been used to demonstrate green chemistry in undergraduate chemistry education.In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C).
591-50-4
491 suppliers
$10.00/1g
768-03-6
110 suppliers
$62.00/250mg

94-41-7
197 suppliers
$10.00/5g
Yield:94-41-7 90%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 110; for 6 h;Heck Reaction;
Steps:
4.2. General procedure for the Heck reaction using the Pd-NHC-MIL-101(Cr) catalyst
General procedure: A conical flask (10 mL) was charged with aryl halide (1.0 mmol), terminal alkene (1.1 mmol), K 2 CO 3 (2 mmol, 0.28 g), Pd-NHC-MIL- 101(Cr) catalyst (0.8 mol %, 6.5 mg) and DMF (5 mL). The mixture was heated in an oil bath at 110 °C. Upon completion of the reac- tion monitored by GC or TLC analysis ( Scheme 2 ), the mixture was filtered and the organic layer was cooled to room temperature and then water added. The organic layer was extracted with ethyl ac- etate (3 ×5 mL) and dried over Na 2 SO 4 . The solvent was removed under reduced pressure and the product was purified by silica gel column chromatography employing n -hexane/ethyl acetate as the eluent, affording the pure corresponding product.
References:
Khalafi-Nezhad, Ali;Niknam, Esmaeil;Panahi, Farhad [Journal of Organometallic Chemistry,2021,vol. 935,art. no. 121676] Location in patent:supporting information

108-86-1
510 suppliers
$10.00/5g

768-03-6
110 suppliers
$62.00/250mg

94-41-7
197 suppliers
$10.00/5g



