
CETYL PALMITATE synthesis
- Product Name:CETYL PALMITATE
- CAS Number:540-10-3
- Molecular formula:C32H64O2
- Molecular Weight:480.85

36653-82-4
521 suppliers
$6.00/100g

540-10-3
304 suppliers
$5.00/5g
Yield:540-10-3 99%
Reaction Conditions:
with sodium bromate;sulfuric acid;sodium bromide in water at 20; for 24 h;
Steps:
General procedure for octyl octanoate (2a)
General procedure: A total of 1.0 g of 1-octanol (7.69 mmol) was taken in a 50-mL round-bottomed flask, to it NaBr 0.523 g (0.66 eq.), NaBrO 3 0.383 g (0.33 eq.), and 10 mL of H 2 O [comprises the bromide and bromate in 2:1 molar ratio] were added[6f]. The reaction mixture was stirred vigorously to dissolve the contents completely. To the above reaction mixture, the aqueous H 2 SO 4 solution (0.5 eq.) was added slowly under stirring over a period of 2.5 h at room temperature (prepared by adding 0.21 mL of 98% H 2 SO 4 to 1 mL of water). The reaction mixture was allowed to stir for up to 24 h. After the completion of reaction, the product was extracted with CH 2 Cl 2 (3 15 mL), the organic layer was dried with Na 2 SO 4 and removal of the solvent obtained octyloctanoate in 98% yield (0.953 g) as colorless liquid. The product was confirmed by GC-MS as well as by NMR.
References:
Reddy, N. Naresh Kumar;Ravi, Chitrakar;Adimurthy, Subbarayappa [Synthetic Communications,2018,vol. 48,# 13,p. 1663 - 1670]

36653-82-4
521 suppliers
$6.00/100g

57-10-3
555 suppliers
$5.00/250mg

540-10-3
304 suppliers
$5.00/5g

57-10-3
555 suppliers
$5.00/250mg

540-10-3
304 suppliers
$5.00/5g

36653-82-4
521 suppliers
$6.00/100g
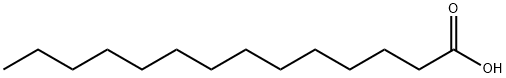
544-63-8
594 suppliers
$5.00/5g

57-10-3
555 suppliers
$5.00/250mg

2599-01-1
59 suppliers
$83.50/10mg

540-10-3
304 suppliers
$5.00/5g

36653-82-4
521 suppliers
$6.00/100g

112-67-4
264 suppliers
$10.00/5mL

540-10-3
304 suppliers
$5.00/5g