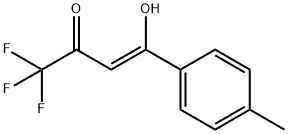
Celecoxib Trifluro Impurity synthesis
- Product Name:Celecoxib Trifluro Impurity
- CAS Number:910303-54-7
- Molecular formula:C11H9F3O2
- Molecular Weight:230.18
Yield:910303-54-7 81%
Reaction Conditions:
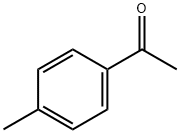
Stage #1: para-methylacetophenonewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 1 h;Inert atmosphere;Claisen Condensation;
Stage #2: ethyl trifluoroacetate, in tetrahydrofuran;mineral oil at 20; for 3.5 h;Inert atmosphere;Claisen Condensation;
Steps:
(Z)-1,1,1-Trifluoro-4-hydroxy-4-(p-tolyl)-3-buten-2-one (1)
4'-Methylacetophenone (100 μL, 753 μmol) was dissolvedin dry tetrahydrofuran (THF) (2.0 mL), and 60% sodium hydride(NaH) (65 mg, 1.63 mmol, 2.2 equivalent (equiv.)) wasadded under ice-cold. After stirring for 1 h under 0 °C, ethyltrifluoroacetate (135 μL, 1.13 mmol, 1.5 equiv.) was added, andthe reaction mixture was stirred for 3.5 h at room temperature.The reaction mixture was evaporated, added ice-water(5 mL), acidified to pH 6 with 1 N hydrochloric acid (HCl),and extracted 3 times with ethyl acetate (EtOAc). The organiclayer was washed with water, dried over magnesium sulfate(MgSO4), filtered, and concentrated. The residue was washedwith n-hexane, and evaporated. The solid was dissolved indichloromethane (CH2Cl2), and evaporated to afford 1 (140 mg,608 μmol, 81%). 1H-NMR (400 MHz, CDCl3) δ: 7.86 (2H, d,J = 8.4 Hz), 7.31 (2H, d, J = 8.4 Hz), 6.55 (1H, s), 2.45 (3H, s).
References:
Yamahana, Hirari;Komiya, Yuki;Takino, Takahisa;Endo, Yoshio;Yamada, Hisatsugu;Asada, Chikako;Uto, Yoshihiro [Chemical and Pharmaceutical Bulletin,2021,vol. 69,# 10,p. 1017 - 1028]