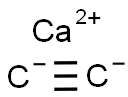
Calcium carbide synthesis
- Product Name:Calcium carbide
- CAS Number:75-20-7
- Molecular formula:C2Ca
- Molecular Weight:64.1
Lime for the reaction is usually made by calcining limestone in a kiln at the plant site. The sources of carbon for the reaction are petroleum coke, metallurgical coke, and anthracite coal. Because impurities in the furnace charge remain in the calcium carbide product, the lime should contain no more than 0.5 percent each of magnesium oxide, aluminum oxide, and iron oxide, and 0.004 percent phosphorus. Also, the coke charge should be low in ash and sulfur. Analyses indicate that 0.2 to 1.0 percent ash and 5 to 6 percent sulfur are typical in petroleum coke. About 991 kilograms (kg) (2,185 pounds [lb]) of lime, 683 kg (1,506 lb) of coke, and 17 to 20 kg (37 to 44 lb) of electrode paste are required to produce 1 megagram (Mg) (2,205 lb) of calcium carbide.

7789-78-8
212 suppliers
$15.00/5g
7440-44-0
309 suppliers
$12.00/25g
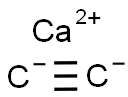
75-20-7
152 suppliers
$47.79/500g
Yield:-
Reaction Conditions:
with hydrogen in neat (no solvent)heating on 1750 - 2000°C under exclusion of air and water;;
References:
[Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Ca: MVol.B1, 62, page 144 - 146]
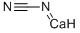
156-62-7
99 suppliers
$29.12/25G
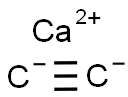
75-20-7
152 suppliers
$47.79/500g

7440-70-2
150 suppliers
$17.00/5g
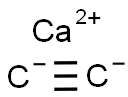
75-20-7
152 suppliers
$47.79/500g

7440-70-2
150 suppliers
$17.00/5g
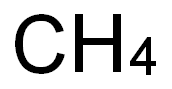
7440-44-0
309 suppliers
$12.00/25g
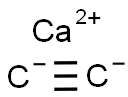
75-20-7
152 suppliers
$47.79/500g