
C 29 synthesis
- Product Name:C 29
- CAS Number:70894-76-7
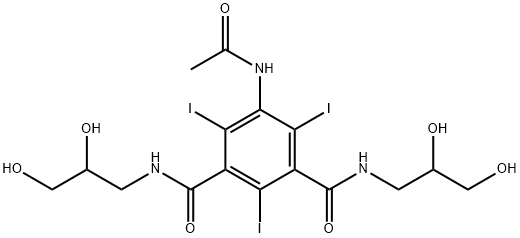
- Molecular formula:C18H24I3N3O8
- Molecular Weight:791.11
31127-80-7
238 suppliers
$24.00/5g

70894-76-7
19 suppliers
inquiry
Yield:70894-76-7 3.8 g (45%)
Steps:
1.d (d)
(d) (+)-5-(N-2-Hydroxyethylacetamido)-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)-isophthalamide The dextrorotatory compound was prepared from (+)-5-acetamido-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)-isophthalamide (8 g) in a similar manner to that described for the preparation of the corresponding laevorotatory compound (Example 1c). Yield: 3.8 g (45%) [α]546 =+5.5°; [α]578 =+4.8° (c=20.0, water). The melting point of the substance is not very characteristic. When heated in a capillary tube, the solid sintered at about 165° and liberated iodine at 205° by further heating. TLC was performed as described in A(b) above. Rf =0.31 in system A 0.31 (endo-isomer) and Rf =0.42 (exo-isomer) in system B. Analysis--Found: C, 27.56; H, 3.08; I, 48.0; N, 5.20. Calc. for C18 H24 I3 N3 O8: C, 27.33; H, 3.06; I, 48.12; N, 5.31.
References:
US4250113,1981,A