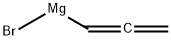
bromo-2-propynyl-Magnesium synthesis
- Product Name:bromo-2-propynyl-Magnesium
- CAS Number:18295-60-8
- Molecular formula:C3H3BrMg
- Molecular Weight:143.27
Yield:-
Reaction Conditions:
mercury dichloride in diethyl ether at 0 - 5; for 1 h;
Steps:
4.A
A 100 mL round bottom flask is charged with magnesium turnings (164 mg, 6.73 mmol) and mercury(II)choride (2.8 mg) and set under nitrogen atmosphere. Ether (1 mL) is added to the solids and the suspension cooled to O0C using ice/water bath. Propargyl bromide (1 mL, 6.73 mmol) was added via syringe dropwise maintaining an internal temperature of 5°C.
References:
MERCK & CO., INC. WO2008/85300, 2008, A1 Location in patent:Page/Page column 46-47

106-96-7
338 suppliers
$30.00/25g
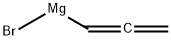
18295-60-8
2 suppliers
inquiry