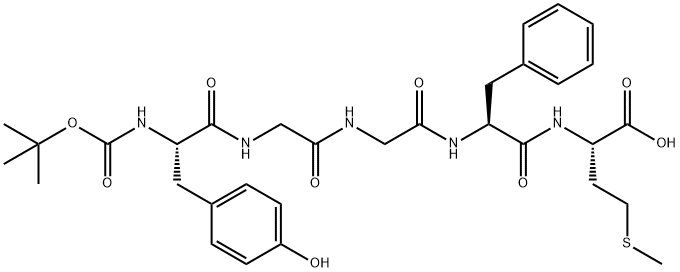
BOC-MET-ENKEPHALIN synthesis
- Product Name:BOC-MET-ENKEPHALIN
- CAS Number:59481-77-5
- Molecular formula:C32H43N5O9S
- Molecular Weight:673.78
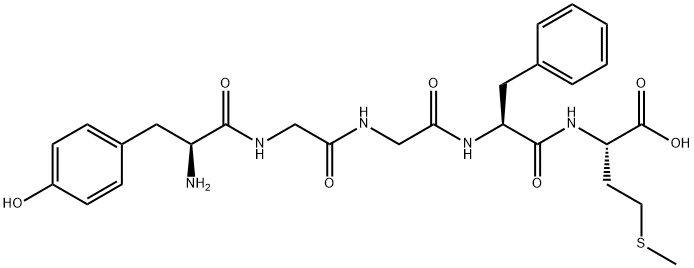
58569-55-4
169 suppliers
$60.00/10mg
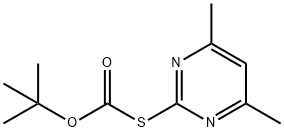
41840-28-2
129 suppliers
$20.69/5G

59481-77-5
30 suppliers
inquiry
Yield:59481-77-5 685 mg (68.7%)
Reaction Conditions:
with triethanolamine in diethyl ether;ethanol;water;acetonitrile;
Steps:
26 Production of Boc-Tyr-Gly-Gly-Phe-Met-OH
EXAMPLE 26 Production of Boc-Tyr-Gly-Gly-Phe-Met-OH In 5 ml of 50% aqueous acetonitrile were dissolved 900 mg of H-Tyr-Gly-Gly-Phe-Met-OH. To the solution was added 0.52 ml of TEA. The mixture was cooled with ice, to which were added 720 mg of Boc-SDP, followed by stirring for 15 hours. The solvent was distilled off. The residue was dissolved in 10 ml of water, and the solution was washed with AcOEt. The aqueous layer was concentrated. To the residue were added 2 ml each of acetic acid and 80% EtOH. The mixtue was placed on a column (3*48 cm) of Sephadex LH-20. Elution was conducted with 80% EtOH, and fractions of 200 to 235 ml were collected and concentrated. The concentrate was dissolved in 30 ml of water and the solution was acidified with AcOH, which was then subjected to extraction with AcOEt. The extract was washed with water and dried over anhydrous sodium sulfate. The solvent was distilled off, and to the residue was added diethyl ether. The resulting powdery product was recovered by filtration and recrystallized from AcOEt. Yield 685 mg (68.7%); m.p. 159°-160° C. (decomp.); [α]D24 -16.8° (c=0.94, DMF); Rf1 =0.21, Rf2 =0.68, Rf3 =0.41. Elemental analysis, for C32 H43 O9 N5 S: Calcd.: C, 57.04; H, 6.43; N, 10.40; S, 4.76; Found: C, 56.58; H, 6.47; N, 10.25; S, 4.76
References:
US4358440,1982,A
![L-Methionine, N-[N-[N-[N-[N-[(1,1-dimethylethoxy)carbonyl]-L-tyrosyl]glycyl]glycyl]-L-phenylalanyl]-, [4-oxo-2-(trifluoromethyl)-4H-1-benzopyran-6-yl]methyl ester (9CI)](/CAS/20210305/GIF/79255-49-5.gif)
79255-49-5
0 suppliers
inquiry

59481-77-5
30 suppliers
inquiry
![L-Methionine, N-[N-[N-[N-[N-[(1,1-dimethylethoxy)carbonyl]-L-tyrosyl]glycyl]glycyl]-L-phenylalanyl]-, 2-amino-2-oxoethyl ester (9CI)](/CAS/20210305/GIF/98229-09-5.gif)
98229-09-5
0 suppliers
inquiry

59481-77-5
30 suppliers
inquiry
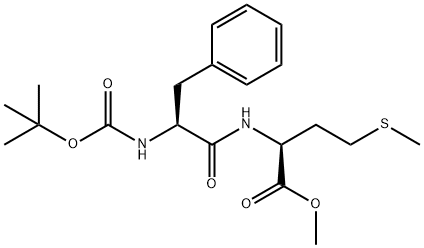
40290-63-9
9 suppliers
inquiry

59481-77-5
30 suppliers
inquiry
![L-Methionine, N-[(1,1-dimethylethoxy)carbonyl]glycylglycyl-L-phenylalanyl-, methyl ester (9CI)](/CAS/20210305/GIF/70035-45-9.gif)
70035-45-9
0 suppliers
inquiry

59481-77-5
30 suppliers
inquiry