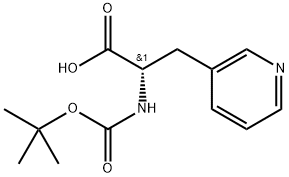
Boc-3-(3-pyridyl)-L-alanine synthesis
- Product Name:Boc-3-(3-pyridyl)-L-alanine
- CAS Number:117142-26-4
- Molecular formula:C13H18N2O4
- Molecular Weight:266.3
24424-99-5
836 suppliers
$13.50/25G

117142-26-4
230 suppliers
$18.00/250mg
Yield:117142-26-4 72%
Reaction Conditions:
with sodium chloride;potassium carbonate;citric acid in 1,4-dioxane;water
Steps:
7 N-t-Butoxycarbonyl-3-(3-pyridyl)-(S)-alanine
PREPARATION 7 N-t-Butoxycarbonyl-3-(3-pyridyl)-(S)-alanine Anhydrous potassium carbonate (7.5 g, 54 mmol) was added to a stirred, ice-cooled suspension of 3-(3-pyridyl)-(S)-alanine (Int. J. Pept. Prot. Res.; 1987, 29, 118; 9 g, 54 mmol) in water (50 ml), followed by the dropwise addition of a solution of di-t-butyl dicarbonate (18 g, 82 mmol) in 1,4-dioxan (25 ml) over 10 minutes. The resulting mixture was allowed to warm to room temperature and stir for 18 hours. The reaction mixture was concentrated by evaporation of the bulk of the 1,4-dioxan under reduced pressure and extracted with ethyl acetate (2*20 ml). The pH of the aqueous solution was adjusted to ca. 3 with solid citric acid, solid sodium chloride added to saturation and extraction with ethyl acetate (5*20 ml) effected. The combined organic extracts were dried (MgSO4), filtered and evaporated to low bulk under reduced pressure, whereupon the product started to crystallise. After chilling at 0° C. for 1 hour, the crystals were collected, washed with ether and dried to give the required product (10.36 g, 72%). Rf 0.34 (SS 13). [α]D25 +10° (c=0.1, CH3 OH). Found: C,58.69; H,7.17; N,10.50. C13 H18 N2 O4 requires C,58.63; H,6.81; N,10.52%.
References:
Pfizer Inc. US5798352, 1998, A