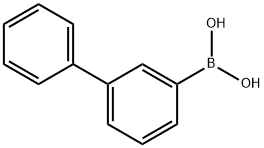
Biphenyl-3-boronic acid synthesis
- Product Name:Biphenyl-3-boronic acid
- CAS Number:5122-95-2
- Molecular formula:C12H11BO2
- Molecular Weight:198.03
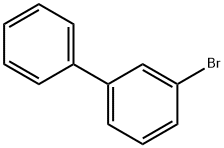
2113-57-7
412 suppliers
$14.00/1g

5122-95-2
332 suppliers
$9.00/1g
Yield:5122-95-2 55%
Reaction Conditions:
Stage #1:3-bromobiphenyl with n-butyllithium in tetrahydrofuran;hexane at -80; for 1 h;Inert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -80 - 20; for 4 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane for 2 h;
Steps:
9.2
Into a 300 mL three-neck flask was put 3.8 g (16 mmol) of 3-bromobiphenyl was put, and the air in the flask was replaced with nitrogen. To the flask was added100 mL of tetrahydrofuran (THF) was added, and this solution was cooled down to -800C. To this solution was added 11 mL (18 mmol) of n-butyllithium (a 1.6 mol/L hexane solution) by being dropped with a syringe. After the dropping was completed, this solution was stirred at the same temperature for 1 hour. After the stir, 2.2 mL (20 mmol) of trimethyl borate was added thereto, and the mixture was stirred for 4 hours while the temperature of the mixture was brought back to a room temperature. After the stir, about 50 mL of dilute hydrochloric acid (1.0 mol/L) was added to the solution, and then the solution was stirred for 2 hours. After stir, the aqueous layer of the mixture was extracted with ethyl acetate and the extracted solution and the organic layer were washed together with a saturated sodium bicarbonate solution and saturated saline. The organic layer was dried with magnesium sulfate, and this mixture was subjected to gravity filtration to obtain a filtrate. The obtained filtrate was concentrated to give an oily substance. Hexane was added to the oily substance, whereby a white solid was precipitated. The obtained solid was collected to give 1.7 g of white powder, which was the object, at a yield of 55%.
References:
SEMICONDUCTOR ENERGY LABORATORY CO., LTD.;SUZUKI, Hiroki;KAWAKAMI, Sachiko;OHSAWA, Nobuharu;SUZUKI, Tsunenori;SEO, Satoshi WO2010/5066, 2010, A1 Location in patent:Page/Page column 178-179

121-43-7
355 suppliers
$13.00/25mL

2113-57-7
412 suppliers
$14.00/1g

5122-95-2
332 suppliers
$9.00/1g
5419-55-6
377 suppliers
$12.00/5g

2113-57-7
412 suppliers
$14.00/1g

5122-95-2
332 suppliers
$9.00/1g
688-74-4
324 suppliers
$10.40/25mL
103068-18-4
34 suppliers
$199.00/50ml

5122-95-2
332 suppliers
$9.00/1g
108-36-1
452 suppliers
$10.00/1g

5122-95-2
332 suppliers
$9.00/1g