
Benzyl chloromethyl ether synthesis
- Product Name:Benzyl chloromethyl ether
- CAS Number:3587-60-8
- Molecular formula:C8H9ClO
- Molecular Weight:156.61
A 1-l, three-necked flask equipped with an overhead mechanical stirrer with a Teflon paddle, gas-inlet tube, thermometer, and a calcium chloride drying tube is charged with 216 g. (2.00 moles) of benzyl alcohol and 66 g. (2.20 moles as CH2O) of paraformaldehyde. The resulting mixture is maintained at 20–25° with a water bath during addition of anhydrous hydrogen chloride at a moderate rate, with stirring. After approximately 2 hours the reaction is complete, as judged by the appearance of two clear homogeneous phases. The layers are separated, and the upper layer is diluted with 800 ml. of pentane and dried over anhydrous magnesium sulfate for 3 hours at 0°, with stirring. The drying agent is removed by filtration, 2–3 g. of anhydrous calcium chloride is added to the filtrate, and the solution is concentrated on a rotary evaporator. The residual liquid, which is nearly pure benzyl chloromethyl ether, is decanted, affording 260 g. (83%) of crude product. This crude benzyl chloromethyl ether, which is suitable for use in some applications, is stored over anhydrous calcium chloride at 0° under an inert atmosphere.
If further purification is desired, just prior to use the crude material (40 g.) may be distilled at approximately 3 mm from anhydrous calcium chloride, affording very pure benzyl chloromethyl ether (35 g.), b.p. 70–71° (3 mm).
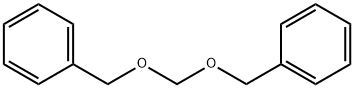
2749-70-4
4 suppliers
inquiry

3587-60-8
321 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
Stage #1: bis(benzyloxy)methanewith magnesium chloride in neat (no solvent) at 20;Inert atmosphere;Schlenk technique;
Stage #2: with thionyl chloride in neat (no solvent) at 0 - 5;Inert atmosphere;Schlenk technique;
Steps:
3.1. General Procedure for the Synthesis of AlkoxymethylHalides (2)
General procedure: Bis-alkoxymethane (20 mmol) was taken with Lewis acidcatalyst (0.01M) in a two-necked round-bottomed flask andstirred for 2-3 minutes at room temperature. The halogenatingagent (22 mmol) was added drop-wise at 0-5oC. Disappearanceof respective alkoxymethane indicated the generationof expected alkoxymethyl halide 2, which was furtherreacted with different nucleophiles as given below
References:
Nisar, Muhammad;Gondal, Humaira Yasmeen;Cheema, Zain Maqsood;Abbasskhan, Ahmed [Letters in Organic Chemistry,2022,vol. 19,# 9,p. 750 - 756]

50-00-0
864 suppliers
$10.00/25g

100-51-6
1396 suppliers
$5.00/100g

3587-60-8
321 suppliers
$10.00/1g
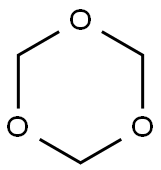
110-88-3
229 suppliers
$13.00/100g

100-51-6
1396 suppliers
$5.00/100g

3587-60-8
321 suppliers
$10.00/1g
![Benzene, [[(methylthio)methoxy]methyl]-](/CAS/20210305/GIF/52132-66-8.gif)
52132-66-8
0 suppliers
inquiry

3587-60-8
321 suppliers
$10.00/1g
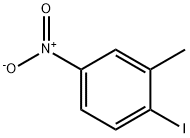
5326-38-5
138 suppliers
$9.00/5g

3587-60-8
321 suppliers
$10.00/1g