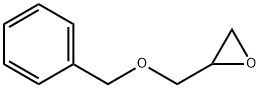
BENZYL GLYCIDYL ETHER synthesis
- Product Name:BENZYL GLYCIDYL ETHER
- CAS Number:2930-05-4
- Molecular formula:C10H12O2
- Molecular Weight:164.2

106-89-8
746 suppliers
$9.00/10 g

100-51-6
1389 suppliers
$5.00/100g

2930-05-4
114 suppliers
$8.00/5g
Yield:2930-05-4 94%
Reaction Conditions:
Stage #1:epichlorohydrin with tetrabutylammomium bromide;potassium hydroxide in water at 20;Cooling with ice;
Stage #2:benzyl alcohol in water at 10 - 20;
Steps:
7.1
A mixture of 50% w/w aqueous potassium hydroxide (30 mL), epichlorohydrin (157 mmol, 20 mL) and tetrabutylammonium bromide (2.35 mmol, 0.75 g) was vigorously stirred at room temperature and cooled in an ice bath. Benzyl alcohol (96.0 mmol, 10 ml) was added dropwise, while maintaining the reaction temperature at about 10° C. by cooling the reaction mixture in an ice bath. The reaction mixture was then allowed to stir at room temperature overnight after which it was poured onto ice/water, and the aqueous phase extracted with diethyl ether. The organic phases were combined and washed with brine to neutrality and dried (Na2SO4). The solution was concentrated under reduced pressure. The residue was purified by flash chromatography (silica, 2-10% ethyl acetate/heptane) to give 14.8 g (94%) of 2-benzyloxymethyl-oxirane. C10H12O2 (164.21), LCMS (ESI): 165.10 (M+H). 1H NMR (CDCl3), 300 MHz), δ 7.26-7.38 (m, 5H), 4.59 (q, 2H), 3.77 (dd, 1H), 3.45 (dd, 1H), 3.19 (m, 1H), 2.80 (t, 1H), 2.63 (dd, 1H).
References:
SANOFI-AVENTIS US2010/75994, 2010, A1 Location in patent:Page/Page column 22-23

556-52-5
258 suppliers
$17.67/1gm:
100-39-0
429 suppliers
$10.00/10 g

2930-05-4
114 suppliers
$8.00/5g
14593-43-2
121 suppliers
$18.00/1g

2930-05-4
114 suppliers
$8.00/5g

556-52-5
258 suppliers
$17.67/1gm:
100-44-7
638 suppliers
$13.50/250G

2930-05-4
114 suppliers
$8.00/5g
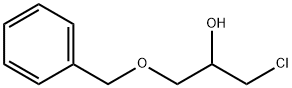
13991-52-1
21 suppliers
inquiry

2930-05-4
114 suppliers
$8.00/5g