beciparcil synthesis
- Product Name:beciparcil
- CAS Number:130782-54-6
- Molecular formula:C12H13NO3S2
- Molecular Weight:283.371
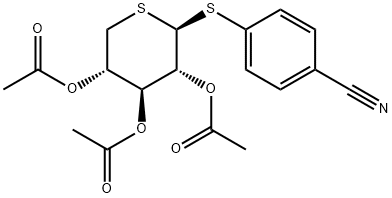
130782-78-4
2 suppliers
inquiry

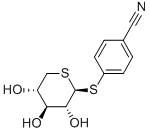
130782-54-6
3 suppliers
inquiry
Yield:130782-54-6 89.7%
Reaction Conditions:
with sodium methylate in methanol;
Steps:
V Preparation of 4-cyanophenyl 1,5-dithio-β-D-xylopyranoside (Example 1)
PREPARATION V Preparation of 4-cyanophenyl 1,5-dithio-β-D-xylopyranoside (Example 1) Under a nitrogen atmosphere, 8.5 g (21.10-3 mol) of 4-cyanophenyl 2,3,4-tri-O-acetyl-1,5-dithio-β-D-xylopyranoside (Example 1a) are suspended in 100 ml of methanol, and 2 ml of sodium methylate (8% w/v of Na in methanol) are then added. The reaction medium is stirred at room temperature until the starting material has completely dissolved (2 hours) and is then neutralized by the addition of Amberlite IR 120 H+ resin. The methanol is evaporated off under reduced pressure; the crude product obtained is recrystallized from an ethanol/water mixture (65/25 v/v) to give 5.3 g (yield: 89.7%) of the expected product. M.p.=175° C. [α]D20° C. =+35.8° (c=0.5; CH3 OH)
References:
US5101048,1992,A
20031-21-4
260 suppliers
$6.00/1g

130782-54-6
3 suppliers
inquiry
![4H-[1,3]Dioxolo[4,5]furo[3,2-d]-1,3,2-dioxathiin, tetrahydro-7,7-dimethyl-, 2-oxide, (2R,4aR,5aR,8aR,8bS)-](/CAS/20210305/GIF/214272-22-7.gif)
214272-22-7
0 suppliers
inquiry

130782-54-6
3 suppliers
inquiry
4539-89-3
0 suppliers
inquiry

130782-54-6
3 suppliers
inquiry
4539-84-8
1 suppliers
inquiry

130782-54-6
3 suppliers
inquiry