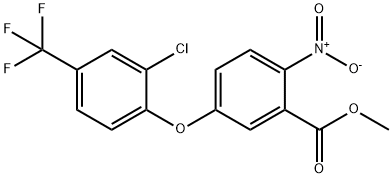
ACIFLUORFEN METHYL ESTER synthesis
- Product Name:ACIFLUORFEN METHYL ESTER
- CAS Number:50594-67-7
- Molecular formula:C15H9ClF3NO5
- Molecular Weight:375.68
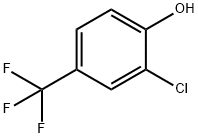
35852-58-5
146 suppliers
$14.00/5g
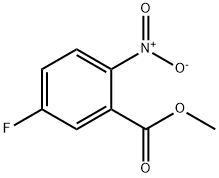
393-85-1
155 suppliers
$9.00/1g

50594-67-7
22 suppliers
$501.05/5MG
Yield:50594-67-7 84%
Reaction Conditions:
with potassium carbonate;dimethyl sulfoxide
Steps:
1.B Methyl 5(2-chloro-4-trifluoromethylphenoxy)2-nitrobenzoate
EXAMPLE 1B Methyl 5(2-chloro-4-trifluoromethylphenoxy)2-nitrobenzoate To a 5-liter, 4-necked flask fitted with a stirrer, thermometer, addition funnel, reflux condenser and drying tube is added dimethyl sulfoxide (200 ml), 2-chloro-4-trifluoromethylphenol) (715 g, 3.64 mole), and anhydrous potassium carbonate (520 g, 3.72 moles). The reaction mixture is stirred at room temperature overnight under a nitrogen atmosphere. Then methyl 5-fluoro-2-nitrobenzoate (724 g, 3.64 moles) is added over a 1 hour period (slight exotherm to 31° C.). After stirring overnight at room temperature, the inorganic solids are removed by filtration and 1700 ml of dimethyl sulfoxide removed under vacuum (1 mm, pot temp. of 85° C., vapor temp. of -40° C.). The residue is diluted with a large volume of carbon tetrachloride and water washed. The organic layer is dried with anhydrous magnesium sulfate and the solvent removed under vacuum to afford 1142 g of methyl 5-(2-chloro-4-trifluoromethylphenoxy)-2-nitrobenzoate (84% yield). Anal. Calcd. for C15 H9 ClF3 NO5: C, 47.90; H, 2.42; Cl, 9.45; F, 15.20; N, 3.73. Found: C, 47.83; H, 2.47; Cl, 9.15; L F, 14.84; N, 3.69.
References:
US4259510,1981,A

35852-58-5
146 suppliers
$14.00/5g
51282-49-6
173 suppliers
$13.00/5g

50594-67-7
22 suppliers
$501.05/5MG
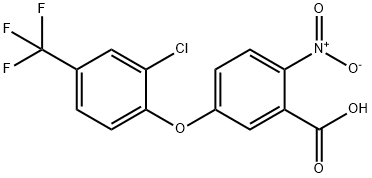
50594-66-6
198 suppliers
$78.00/1g

50594-67-7
22 suppliers
$501.05/5MG

328-84-7
376 suppliers
$5.00/5g

50594-67-7
22 suppliers
$501.05/5MG
99-06-9
502 suppliers
$5.00/25g

50594-67-7
22 suppliers
$501.05/5MG