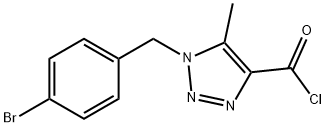
1-(4-BROMOBENZYL)-5-METHYL-1H-[1,2,3]-TRIAZOLE-4-CARBONYL CHLORIDE synthesis
- Product Name:1-(4-BROMOBENZYL)-5-METHYL-1H-[1,2,3]-TRIAZOLE-4-CARBONYL CHLORIDE
- CAS Number:952182-50-2
- Molecular formula:C11H9BrClN3O
- Molecular Weight:314.57
845885-94-1
20 suppliers
$25.00/50mg
![1-(4-BROMOBENZYL)-5-METHYL-1H-[1,2,3]-TRIAZOLE-4-CARBONYL CHLORIDE](/StructureFile/ChemBookStructure9/GIF/CB1387092.gif)
952182-50-2
8 suppliers
$244.00/500mg
Yield:952182-50-2 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 1 h;
Steps:
1-(4-Bromo-benzyl)-5-methyl-1 H-[1 ,2,3]triazole-4-carboxylic acid [5-chloro-2-(1 H- tetrazol-5-yl)-phenyl]-amide (3)To a stirred suspension of the commercial 1 -(4-bromo-benzyl)-5-methyl-1 H- [1 ,2,3]triazole-4-carboxylic acid (0.200 g, 1 eq) in DCM (15 ml), an excess of oxalyl chloride (1 ml) is added drop wise at 00C, followed by 1 -2 drops of dry DMF. The resulting yellow solution is allowed to reach room temperature spontaneously and then stirred at room temperature until starting material disappears completely on TLC (~1 hour). The mixture is then evaporated to dryness under vacuum and the residue is taken up in DCM and washed with cold aqueous NaHCO3. The organic phase, dried
References:
WO2008/138917,2008,A1 Location in patent:Page/Page column 25-26