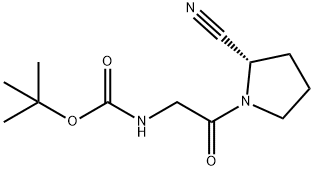
(S)-tert-butyl 2-(2-cyanopyrrolidin-1-yl)-2-oxoethylcarbamate synthesis
- Product Name:(S)-tert-butyl 2-(2-cyanopyrrolidin-1-yl)-2-oxoethylcarbamate
- CAS Number:952023-06-2
- Molecular formula:C12H19N3O3
- Molecular Weight:253.3
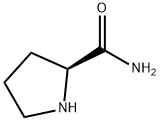
![L-Prolinamide, N-[(1,1-dimethylethoxy)carbonyl]glycyl-](/CAS/20210111/GIF/52293-28-4.gif)
52293-28-4
0 suppliers
inquiry

952023-06-2
21 suppliers
inquiry
Yield:952023-06-2 84.9%
Reaction Conditions:
with pyridine;1H-imidazole;trichlorophosphate at -35 - 20; for 1.5 h;
Steps:
1.2
2) Preparation of [2-(2-cyano-pyrrolidin-1-yl)-2-oxo-ethyl]-carbamic acid tert-butyl ester 1f In dry three-neck flask under a nitrogen atmosphere, 286 mL pyridine, [2-(2-carbamoyl-pyrrolidin-1-yl)-2-oxo-ethyl]-carbamic acid tert-butyl ester (13.5 g, 49.8 mmol) and imidazole (7.11 g, 104.6 mmol) were added in order, phosphorus oxychloride (19 mL, 204.2 mmol) was added at -35 under stirring. Upon completion of the addition, the reaction mixture was stirred for 1 hour at -35 , then naturally raised to room temperature and reacted for 0.5 hour. Pyridine was evaporated and the reaction mixture was extracted with ethyl acetate (200 mL×3). The combined organic phase was washed with 50 mL saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to give the title compound [2-(2-cyano-pyrrolidin-1-yl)-2-oxo-ethyl]-carbamic acid tert-butyl ester 1f (10.7 g, yield 84.9%) as a white powder. MS m/z (ESI) : 254.3[M+1].
References:
EP2006284,2008,A1 Location in patent:Page/Page column 18-19
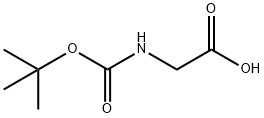
4530-20-5
540 suppliers
$5.00/5G

952023-06-2
21 suppliers
inquiry
7531-52-4
667 suppliers
$6.00/5g

952023-06-2
21 suppliers
inquiry
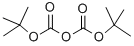
24424-99-5
832 suppliers
$13.50/25G

952023-06-2
21 suppliers
inquiry
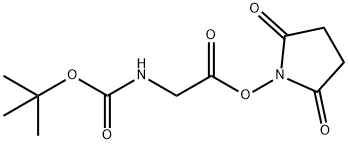
3392-07-2
134 suppliers
$30.00/5G

952023-06-2
21 suppliers
inquiry