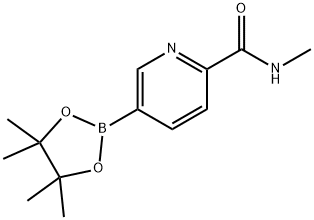
2-(N-Methylaminocarbonyl)-5-pyridineboronic acid pincol ester synthesis
- Product Name:2-(N-Methylaminocarbonyl)-5-pyridineboronic acid pincol ester
- CAS Number:945863-21-8
- Molecular formula:C13H19BN2O3
- Molecular Weight:262.11
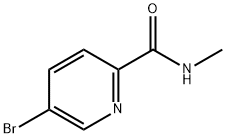
845305-87-5
58 suppliers
$16.00/100mg
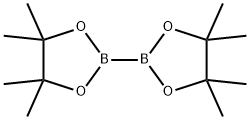
73183-34-3
576 suppliers
$6.00/5g

945863-21-8
63 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 110; for 1 h;Inert atmosphere;Microwave irradiation;Suzuki Coupling;
Steps:
6
Example 6; Preparation of 5-(2-Acetamidothiazolo[5,4-Z>]pyridin-5-yl)-N- methylpicolinamide (6); PcIdPPfCI2-CH2CI2 KOAc, 1 ,4-dioxane microwave, 110 0C Example 1 ditbpfPdCI2, Cs2CO3 NaHCO3(Sat.), DMF, microwave, 110-120 0C[0381] A mixture of 5-bromo-N-methylpicolinamide (320 mg, 1.48 mmol), bispinacolatodiboron (752 mg, 2.96 mmol), PddppfCl2-DCM (cat.), KOAc (436 mg, 4.44 mmol) in 1,4-dioxane (5 mL, degassed) was heated at 110 0C in a microwave for 1 h. To this mixture was then added Example 1 (608 mg, 2.22 mmol), ditbpfPdC12 (cat.), Cs2CO3 (1.45 g, 4.44 mmol), NaHCO3 (sat., 1 mL) and DMF (dry, degassed, 1 mL). It was heated in a microwave at 1100C for 1 h, then at 120 0C for another hour. The reaction mixture was purified by HPLC to give the titled compound as a white solid. 1H NMR (400 MHz, DMSO-rfβ) δ ppm 12.53 (s, IH), 9.28 (d, J=4 Hz, IH), 8.79 (m, IH), 8.61 (dd, J=8 Hz, IH), 8.18 (m, 2H), 8.08 (d, J=8 Hz, IH), 2.78 (d, J=4 Hz, IH), 2.18 (s, 3H); [M+H] calc'd for Ci5Hi3N5O2S, 328; found, 328.
References:
WO2010/8847,2010,A2 Location in patent:Page/Page column 125-126