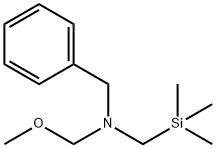
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine synthesis
- Product Name:N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
- CAS Number:93102-05-7
- Molecular formula:C13H23NOSi
- Molecular Weight:237.41

50-00-0
862 suppliers
$10.00/25g
![N-[(Trimethylsilyl)methyl]benzylamine](/CAS/GIF/53215-95-5.gif)
53215-95-5
149 suppliers
$11.00/1g

93102-05-7
302 suppliers
$6.00/1g
Yield:93102-05-7 97.5%
Reaction Conditions:
Stage #1: formaldehyd;N-(trimethylsilylmethyl)benzylamine in water at 0;
Stage #2: with potassium carbonate in water at 20; for 2 h;
Steps:
Intermediate P16: N-Benzyl-1 -methoxy-N-[(trimethylsilyl)methyl]methanamine
To the solution of formaldehyde (37%, 16.816 g, 207.19 mmol) at 0°C N-benzylo- 1 -(trimethylsilyl)methanamine (Intermediate P15, 28.588 g, 144.89 mmol) was added dropwise. To the mixture solid potassium carbonate (16.182 g, 115.91 mmol) was added and the whole was stirred at room temperature for 2 hours. Water (50 ml) was added and the mixture was extracted with diethyl ether (2 x 50 ml). Organic layer was washed with brine and dried over sodium sulphate. Solvent and drying agent were removed to obtain 33.551 g of the title product in the form of a yellow oil (yield 97.5%). 1H NMR (300 MHz, CDCl3): δ 7.35 - 7.18 (m, 5H), 3.99 (s, 2H), 3.75 (s, 2H), 3.22 (s, 3H), 2.18 (s, 2H), 0.13 (s, 9H).
References:
WO2014/24125,2014,A1 Location in patent:Page/Page column 24-25

67-56-1
746 suppliers
$9.00/25ml

50-00-0
862 suppliers
$10.00/25g
![N-[(Trimethylsilyl)methyl]benzylamine](/CAS/GIF/53215-95-5.gif)
53215-95-5
149 suppliers
$11.00/1g

93102-05-7
302 suppliers
$6.00/1g

67-56-1
746 suppliers
$9.00/25ml
![N-[(Trimethylsilyl)methyl]benzylamine](/CAS/GIF/53215-95-5.gif)
53215-95-5
149 suppliers
$11.00/1g

93102-05-7
302 suppliers
$6.00/1g
![N-[(Trimethylsilyl)methyl]benzylamine](/CAS/GIF/53215-95-5.gif)
53215-95-5
149 suppliers
$11.00/1g
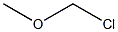
107-30-2
1 suppliers
$110.00/2.5g

93102-05-7
302 suppliers
$6.00/1g