
(9-phenyl-9H-carbazol-2-yl)boronic acid synthesis
- Product Name:(9-phenyl-9H-carbazol-2-yl)boronic acid
- CAS Number:1001911-63-2
- Molecular formula:C18H14BNO2
- Molecular Weight:287.12


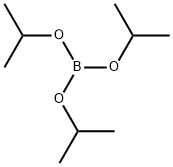
Sub 1-3-1 (6.4g, 20mmol) was dissolved in anhydrous Ether, lowering the temperature of the reaction to -78°C, n-BuLi (2.5M in hexane) (1.4g, 22mmol) was added dropwise slowly, and after, the reaction It was stirred for 30 minutes. After lowering the temperature of the reaction back to -78°C dropwise Triisopropyl borate (5.6g, 30mmol). Stirring at room temperature, diluted with water and it binds the 2N HCl. After completion of reaction, ethyl acetate and water, dried over MgSO4 and the organic layer was extracted and recrystallized silicagel column and the resulting organic material and then concentrated to a Sub 1-4-1 4.5g (yield: 78%) was obtained.
5419-55-6
374 suppliers
$12.00/5g
94994-62-4
187 suppliers
$7.00/250mg

1001911-63-2
183 suppliers
$5.00/100mg
Yield:1001911-63-2 95%
Reaction Conditions:
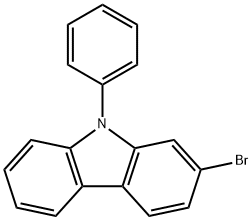
Stage #1: 9-(phenyl)-2-bromo-9H-carbazolewith n-butyllithium in tetrahydrofuran at -75 - 0; for 1 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran at -70 - 20; for 12 h;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;water; for 0.5 h;Inert atmosphere;Temperature;
Steps:
2 Example 2. Synthesis of Intermediate 2
After nitrogen was blown into the reactor and completely replaced with nitrogen, 500g (6.94mmole) of Anhydrous THF was added, and 100g (0.310mmole) of 2-Bromophenylcarbazole was added to completely dissolve it.When completely dissolved, set the outside of the reactor to -75 or less through Dry Ice + Acetone Bath, and then slowly add 94.6g (0.341mmole) of 2.5M n-BuLi dropwise while maintaining the temperature inside the reactor around -70. Was stirred at 0° C. for 1 hour.Thereafter, the temperature of the reactant was lowered to -70°C, and 70g (0.372mmole) of Triiospropyl borate (TIPB) was added dropwise, followed by stirring at room temperature for 12 hours. When the reaction was completed, 4N-HCl aqueous solution was added and stirred for 30 minutes, extracted with Ethyl Acetate (EA), concentrated in an organic solvent, and recrystallized with Hexane to obtain 78 g of a product. The yield is 85% and the purity of the product is more than 99.5%.In order to synthesize the final product, a large amount of intermediates was required, so a sufficient amount to be used in the next reaction was secured through scale Up. Fig. 3).
References:
KR2020/48965,2020,A Location in patent:Paragraph 0089-0100

121-43-7
353 suppliers
$14.00/25mL

94994-62-4
187 suppliers
$7.00/250mg

1001911-63-2
183 suppliers
$5.00/100mg

94994-62-4
187 suppliers
$7.00/250mg

1001911-63-2
183 suppliers
$5.00/100mg

121-43-7
353 suppliers
$14.00/25mL

94994-62-4
187 suppliers
$7.00/250mg

7732-18-5
466 suppliers
$13.50/100ML

1001911-63-2
183 suppliers
$5.00/100mg

7647-01-0
11 suppliers
$10.00/10g

121-43-7
353 suppliers
$14.00/25mL

94994-62-4
187 suppliers
$7.00/250mg

1001911-63-2
183 suppliers
$5.00/100mg