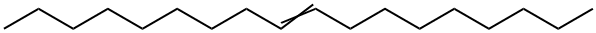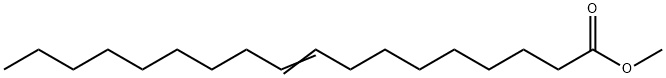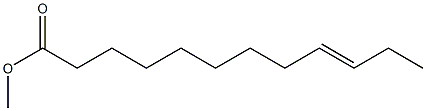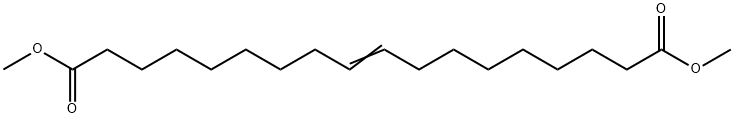
9-Octadecenedioic acid, dimethyl ester synthesis
- Product Name:9-Octadecenedioic acid, dimethyl ester
- CAS Number:13481-97-5
- Molecular formula:C20H36O4
- Molecular Weight:340.5
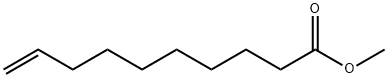
25601-41-6
45 suppliers
inquiry

13481-97-5
0 suppliers
inquiry
Yield:13481-97-5 80.9%
Reaction Conditions:
with Mo(N-2,6-’Pr2-C5H3)(CHCMe2Ph)(2,5-dimeth-ylpyrrolide)(O-2,6-Ph2C5H3) at 50;Inert atmosphere;Molecular sieve;Glovebox;Reagent/catalyst;Temperature;
Steps:
Synthesis of 9-ODDAME:
In a N2-filled glove box, a 1 -L round-bottomed flask equipped with a magnetic stir bar was charged with 250 g 9-DAME that had been dried via passage through a column of activated alumina and then stored over activated 4 A molecular sieves. A solution of X004 was prepared by combining 40.1 mg Mo(NAr) (CHCMe2Ph)(Me2pyr)2 and 16.7 mg 2,6-diphenylphenol in 1 mL of toluene followed by stirring the solution at ambient temperature for 30 minutes. The catalyst solution was added to the ester and the mixture was stirred open to the glove box atmosphere for 6 hours, after which time the mixture was placed under dynamic vacuum for 2 hours during which time gas evolution was observed. Afier standing overnight, the flask was removed from the glove box after an inlet adapter with a needle valve was fitted. The mixture was melted in a 50°C. silicone oil bath and placed under dynamic vacuum for1 hour during which time more gas evolution was observed. The observed GC conversion was 92% (18,400 TON). Neutral activated alumina (12.5 g) was added and the mixture stirred for 30 minutes and then the alumina was removed by filtration. The light components of the mixture were removed by vacuum distillation (120° C. at 0.3 mm Hg) and then the bottoms were again treated with 12.5 g of neutral activated alumina to remove a green colored impurity. The isolated yield was 186.91 g (80.9% yield).
References:
US2014/275595,2014,A1 Location in patent:Paragraph 0170
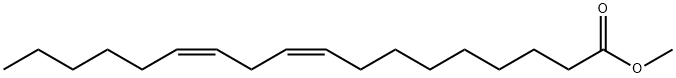
112-63-0
260 suppliers
$15.00/5g

13481-97-5
0 suppliers
inquiry