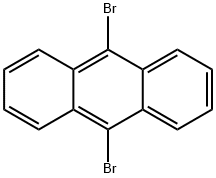
9,10-Dibromoanthracene synthesis
- Product Name:9,10-Dibromoanthracene
- CAS Number:523-27-3
- Molecular formula:C14H8Br2
- Molecular Weight:336.02
77-48-5
541 suppliers
$11.00/10g

120-12-7
330 suppliers
$10.00/5g
1564-64-3
445 suppliers
$8.00/5g

523-27-3
392 suppliers
$6.00/5g
Yield:-
Reaction Conditions:
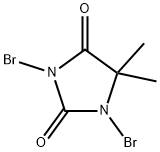
Stage #1: 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;anthracene in ethyl acetate; for 2.5 h;Refluxing;
Stage #2: in tetrahydrofuran;
Steps:
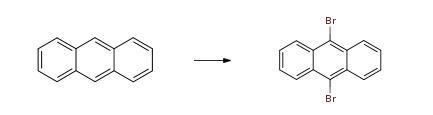
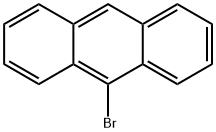
Anthracene (50 g, 0.28 mol, 1 eq) and 1,3-dibromo-5,5-dimethylhydantoin (38.15 g, 0.133 mol, 0.475 eq) were combined in a 500 mL of ethyl acetate and brought to reflux. After about 5 min of reflux, the mixture became homogeneous, and yellow, and stayed light in color throughout the 2.5 h that it was refluxed. The reaction mixture allowed to cool and then washed with water to remove the 5,5-dimethylhydantoin. When water was added, the mixture turned greenish-blue and the color persisted through additional aqueous washes. The organic layer was dried over Na2SO4 and the greenish color gradually disappeared, and the mixture became orange-brown. The liquor was concentrated to dryness, to yield a brown solid residue. The solid was dissolved in hot THF (about 50 mL) and then slowly it was precipitated again with addition of acetonitrile (about 200 mL). The yellowish solid was isolated by filtration (36.4 g) and the process was repeated twice to yield two more crops of solid (total 23.8 g). The 3 crops were combined and analyzed by high performance liquid-chromatography (HPLC). The analysis indicated that only 89% of the reaction product was the desired 9-bromoanthracene, 9.3% was unreacted anthracene and 1% was the undesirable 9,10-dibromoanthracene. The 9,10-dibromoanthracene could not be removed by recrystallization.
References:
US2005/245752,2005,A1 Location in patent:Page/Page column 8

120-12-7
330 suppliers
$10.00/5g

523-27-3
392 suppliers
$6.00/5g

1564-64-3
445 suppliers
$8.00/5g

523-27-3
392 suppliers
$6.00/5g

67-56-1
756 suppliers
$9.00/25ml

120-12-7
330 suppliers
$10.00/5g
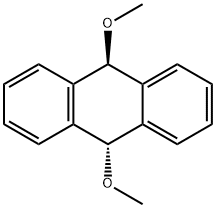
31750-30-8
0 suppliers
inquiry

523-27-3
392 suppliers
$6.00/5g
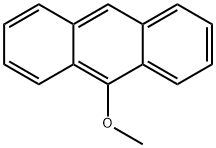
2395-96-2
5 suppliers
inquiry
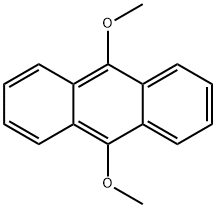
2395-97-3
7 suppliers
$134.00/5mg