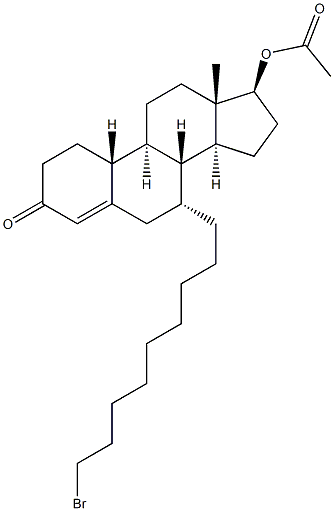
(7alpha,17beta)-17-(Acetyloxy)-7-(9-bromononyl)estr-4-en-3-one synthesis
- Product Name:(7alpha,17beta)-17-(Acetyloxy)-7-(9-bromononyl)estr-4-en-3-one
- CAS Number:875573-63-0
- Molecular formula:C29H45BrO3
- Molecular Weight:521.57
![7β-Acetyloxy-7α-[9-(dimethyl(tert-butylsilyloxy)nonyl]estr-4-en-3-one](/CAS/20211123/GIF/875573-60-7.gif)
875573-60-7
15 suppliers
inquiry

875573-63-0
30 suppliers
$1066.00/1g
Yield:-
Reaction Conditions:
with dibromotriphenylphosphorane in acetonitrile at 10 - 20; for 0.5 h;Product distribution / selectivity;
Steps:
7 Preparation of Cp 9341 from Cp 9295-Direct Synthesis (Depicted in FIG. 4)
Example 7 Preparation of Cp 9341 from Cp 9295-Direct Synthesis (Depicted in FIG. 4) 190 grams of crude Cp 9295 (17β-hydroxy-7α-[9-[[1,1-dimethylethyl)dimethylsilyl]oxy]nonyl]-estr-4-en-3-one acetate), prepared from 75 grams of 6-dehydronandrolone acetate using conditions of Example 4, are dissolved in 100 grams of acetonitrile and added to a suspension at 10-12° C. of triphenylphosphine dibromide (prepared by addition of 140 grams of bromine to 240 grams of triphenylphosphine in 1200 grams of acetonitrile). The temperature is allowed to rise to ambient temperature and after 0.5 hours conversion into Cp 9341 is complete by HPLC. The suspension is diluted with 1000 grams of toluene, neutralised with ca. 112 grams of ammonium hydroxide solution 25%, and the phases are separated. The upper (organic) phase is washed with water (100 g) and then evaporated to an oily residue. The residue is taken up in toluene and stirred at 5-10° C. in order to precipitate most of the triphenylphosphine oxide by-product. The by-product is filtered off and rinsed with toluene and the filtrate purified by chromatography on silica gel (1250 grams) eluding with toluene. Weight of pure Cp 9341 obtained: 87 g.
References:
US2006/30552,2006,A1 Location in patent:Page/Page column 24
![Estr-4-en-3-one, 17-(acetyloxy)-7-[9-[(methylsulfonyl)oxy]nonyl]-, (7α,17β)-](/CAS/20210305/GIF/875573-65-2.gif)
875573-65-2
1 suppliers
inquiry

875573-63-0
30 suppliers
$1066.00/1g

55362-80-6
285 suppliers
$10.00/1g

875573-63-0
30 suppliers
$1066.00/1g
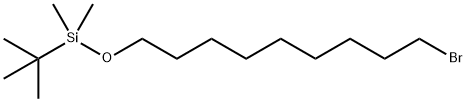
149051-24-1
19 suppliers
inquiry

875573-63-0
30 suppliers
$1066.00/1g
