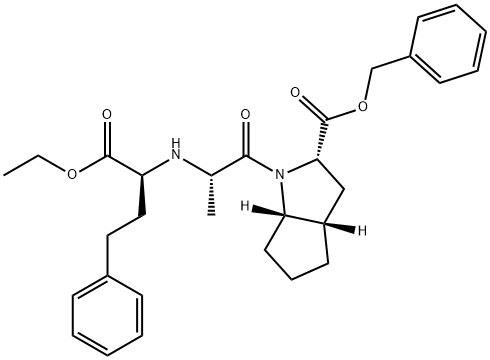
2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER synthesis
- Product Name:2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER
- CAS Number:87269-88-3
- Molecular formula:C30H38N2O5
- Molecular Weight:506.63
Yield:-
Reaction Conditions:
Stage #1: (2S,3aS,6aS)-benzyl octahydrocyclopenta[b]pyrrole-2-carboxylate 4-toluenesulfonate (1:1)with triethylamine in dichloromethane at 0; for 0.5 h;
Stage #2: N-[(S)-1-carbethoxy-3-phenyl-propyl]-S-alaninoyl chloride hydrochloridewith 1H-imidazole in dichloromethane at 0 - 20; for 4.5 h;
Steps:
9
Example 9; Preparation of (2S,3aS,6aS)-octahydrocyclopenta[b]pyrrole-2-carboxylic acid,1 -[(2S)-2-[[(1 S)-1 -(ethoxycarbonyl)-3-phenylpropyl]amino]-1 -oxopropyl], phenylmethyl ester from (2S,3aS,6aS)-benzyl octahydrocyclopenta[b]pyrrole-2- carboxylate; The toluenesulfonate salt prepared in Example 7 (6.00 g, 14.4 mmol) was suspended in CH2CI2 (60 ml.) and triethylamine (1.46 g, 14.4 mmol) was added at 00C. The slurry was stirred for 30 min and then imidazole (2.94 g, 43.1 mmol) was added in small portions, followed by N-[(S)-1-(ethoxycarbonyl)-3-phenyl-propyl]-L-alanylchloride HCI prepared in Example 8 (5.28 g ,15.8 mmol). The reaction mixture was stirred for 2 h at 00C and then
References:
WO2009/50041,2009,A1 Location in patent:Page/Page column 14-15
15121-89-8
95 suppliers
$6.00/1g
![2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER](/StructureFile/ChemBookStructure22/GIF/CB8986018.gif)
87269-88-3
11 suppliers
inquiry
![Benzenebutanoic acid, α-[[(1S)-1-methyl-2-oxo-2-(phenylmethoxy)ethyl]amino]-γ-oxo-, ethyl ester, (αS)-](/CAS/20210305/GIF/87269-98-5.gif)
87269-98-5
1 suppliers
inquiry
![2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER](/StructureFile/ChemBookStructure22/GIF/CB8986018.gif)
87269-88-3
11 suppliers
inquiry

![Benzenebutanoic acid, α-[[(1S)-2-chloro-1-methyl-2-oxoethyl]amino]-, ethyl ester, hydrochloride (αS)-](/CAS/20211123/GIF/114192-42-6.gif)
![Cyclopenta[b]pyrrole-2-carboxylic acid, octahydro-, (2S,3aS,6aS)-, phenylmethyl ester, 4-methylbenzenesulfonate (1:1)](/CAS/20211123/GIF/852546-84-0.gif)
![(S,S,S)-2-Azabicyclo[3,3,0]-octane-carboxylic acid benzylester hydrochloride](/CAS/GIF/93779-31-8.gif)
![N-[(S)-(+)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanine](/CAS/GIF/82717-96-2.gif)
![Ethyl (S)-2-[(S)-4-methyl-2,5-dioxo-1,3-oxazolidin-3-yl]-4-phenylbutyrate](/CAS/GIF/84793-24-8.gif)