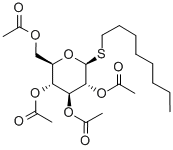
Octyl2,3,4,6-tetra-O-acetyl-b-D-thioglucopyranoside synthesis
- Product Name:Octyl2,3,4,6-tetra-O-acetyl-b-D-thioglucopyranoside
- CAS Number:85618-26-4
- Molecular formula:C22H36O9S
- Molecular Weight:476.58
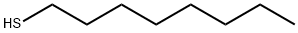
111-88-6
213 suppliers
$15.00/25g
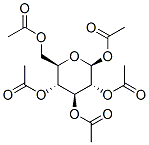
604-69-3
387 suppliers
$10.00/25g

85618-26-4
22 suppliers
$75.00/250mg
Yield:-
Reaction Conditions:
with indium(III) bromide in chloroform;Reflux;
Steps:
10
General procedure: Peracetyl-β-D-glycopyranose (1-5, 10mmol), InBr3 (106mg, 0.3mmol, 0.03eq), and alkane-1-thiol (20mmol, 2eq) were suspended in CHCl3 (7mL). The reaction mixture was heated to reflux with magnetic stirring. The course of the reaction was followed by TLC (hexane:EtOAc 1:1). After 1 hour the peracetate starting material could no longer be detected by TLC. The reaction was cooled to RT, diluted with CH2Cl2 (20mL), and this solution was applied to a short column (4cm×12cm) of SiO2. The HOAc that was formed from the reaction and the excess alkane-1-thiol were eluted with hexanes. The desired thio-glycoside peracetate was eluted with hexanes:EtOAc 7:3. The fractions containing the desired material were combined, concentrated in vacuo, and the intermediate peracetates (12-14, 16-28) were characterized by 1D, 2D, 1H-NMR and 13CNMR in CDCl3 (in Tables in Supplementary data)
References:
Szabó, Lajos Z.;Hanrahan, Dillon J.;Jones, Evan M.;Martin, Erin;Pemberton, Jeanne E.;Polt, Robin [Carbohydrate Research,2016,vol. 422,p. 1 - 4]
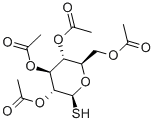
19879-84-6
154 suppliers
$13.00/250mg

111-66-0
159 suppliers
$10.00/10ml

85618-26-4
22 suppliers
$75.00/250mg

111-83-1
500 suppliers
$12.00/25g
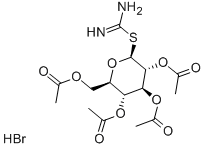
40591-65-9
35 suppliers
$739.20/1G

85618-26-4
22 suppliers
$75.00/250mg
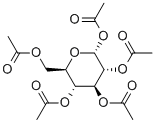
604-68-2
334 suppliers
$6.00/25g

85618-26-4
22 suppliers
$75.00/250mg