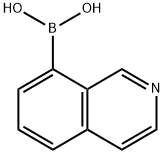
8-isoquinolinyl-boronic acid synthesis
- Product Name:8-isoquinolinyl-boronic acid
- CAS Number:721401-43-0
- Molecular formula:C9H8BNO2
- Molecular Weight:172.98
63927-22-0
250 suppliers
$7.00/250mg

121-43-7
353 suppliers
$13.00/25mL

721401-43-0
88 suppliers
$45.00/25mg
Yield: 41%
Reaction Conditions:
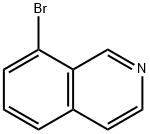
Stage #1:8-bromo-isoquinoline with n-butyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran at 0; for 1 h;
Steps:
27 Example 27. Synthesis of 3-(isoq u inol i n-8-yloxy)-4-methyl-N-(3-(4-methyl-H-imidazol-1 -yl)-5-(4-methylpiperazin-1 -yl)phenyl)benzamide.
To a solution of 8-bromoisoquinoline (2.0 g, 9.6 mmol) in THE (40 mL) was added dropwise n-BuLi (2.5 M, 4.2 mL, 10.6 mmol) at -78 °C under nitrogen. After 1 hour, B(OMe)3 (2.0 g, 19.2 mmol) was added to the reaction and the mixture was warmed to 0 °C for 1 hour. The reaction was quenched by aqueous NaHCO3 andextracted with EtOAc 3 times. The combined organic layers were washed with brine, dried over Na2SO4 and concentrated. The residue was purified by silica gel column chromatography to give 75 (680 mg, 41%) as a slightly yellow solid. LCMS (m/z: m+1):174.1.
References:
CARDIO THERAPEUTICS PTY LTD;TREUTLEIN, Herbert;ZENG, Jun;DIXON, Ian;JAMES, Ian;PALMER, James T WO2018/165718, 2018, A1 Location in patent:Page/Page column 93

63927-22-0
250 suppliers
$7.00/250mg

721401-43-0
88 suppliers
$45.00/25mg