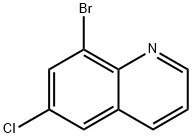
8-BROMO-6-CHLOROQUINOLINE synthesis
- Product Name:8-BROMO-6-CHLOROQUINOLINE
- CAS Number:16567-11-6
- Molecular formula:C9H5BrClN
- Molecular Weight:242.5
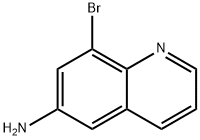
858420-01-6
8 suppliers
inquiry

16567-11-6
50 suppliers
$64.00/250mg
Yield:16567-11-6 287 mg
Reaction Conditions:
with hydrogenchloride;copper(l) chloride;sodium nitrite in water at 0; for 0.85 h;
Steps:
3 Step 3.Preparation of 8-bromo-6-chloroquinoline
Step 3.
Preparation of 8-bromo-6-chloroquinoline
8-Bromoquinolin-6-amine (300 mg, 1.34 mmol) was taken up in concentrated hydrochloric acid (8 ml) and cooled to 0° C. (ice bath).
Sodium nitrite (1.86 gm, 26.9 mmol) was added in three equal portions over 10 minutes.
The mixture was removed from the cooling bath and copper (I) chloride (3.33 g, 33.6 mmol) was added in 3 portions, over about 6 minutes.
On stirring a green-black rising foam developed.
Stirring was continued for 45 minutes and then the reaction mixture was cooled to 0° C. (ice bath).
A mixture of ice water (75 ml) and ammonium hydroxide (75 ml) was added with vigorous stirring.
Dichloromethane (150 ml) was added and the material was shaken in a reparatory funnel.
The organic phase was collected and shaken with an equal volume of brine.
The aqueous phases were back extracted with dichloromethane (2*120 ml).
The organics were combined, dried over magnesium sulfate, filtered and concentrated in vacuo.
The crude product was purified by preparative thin layer chromatography (2 plates), eluting first with 1% methanol/dichloromethane and then re-developing the plate with 25% ethyl acetate/hexane.
The product band was collected, providing the desired product as a light yellow-white solid (287 mg).
(M+H)+=242/244 m/e.
References:
US2013/150360,2013,A1 Location in patent:Paragraph 0267; 0268
120287-30-1
38 suppliers
inquiry

16567-11-6
50 suppliers
$64.00/250mg