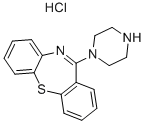
11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE synthesis
- Product Name:11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE
- CAS Number:753475-15-9
- Molecular formula:C17H18ClN3S
- Molecular Weight:0
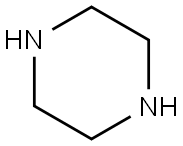
110-85-0
560 suppliers
$5.00/5G
![10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine](/CAS/GIF/3159-07-7.gif)
3159-07-7
348 suppliers
$12.00/5g
![11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE](/CAS/GIF/753475-15-9.gif)
753475-15-9
17 suppliers
$197.00/1mL
Yield:753475-15-9 90%
Reaction Conditions:
Stage #1: dibenzo[b,f][1,4]thiazepin-11-onewith trichlorophosphate in toluene at 10; for 4 h;Heating / reflux;
Stage #2: with potassium carbonate in water;toluene at 15;
Stage #3: piperazinewith hydrogenchloridemore than 3 stages;
Steps:
a
a) ll-piperazinyl-dibenzo[b,f][l,4] thiazepine dihydrochloride (IV)Dibenzo[b,fj[l,4] thiazepin-l l-[10H]one 100 gm, phosphorus oxychloride 125 ml, N5N- dimethyl aniline 33 ml in toluene 500 ml were refluxed for 4 hour. Toluene was distilled off under vacuum to obtain residue. The residue was dissolved in 250 ml toluene and the reaction mixture was cooled to 100C. Aqueous Potassium carbonate solution (100 g in 200 ml water) was added to the reaction mixture below 150C. The toluene layer was separated and refluxed with piperazine 100 g for 24 hour. The reaction mixture was cooled and filtered to remove unreacted piperazine. Toluene was distilled off under vacuum. The residue was dissolved in 390 ml of isopropyl alcohol. 100 ml of 33% ethanolic hydrochloride was added to the reaction mixture at 25-35° C. White solid obtained was filtered, washed with 100 ml ethanol and dried at 60-700C. Yield : 138 g (90 %).
References:
WO2007/4234,2007,A1 Location in patent:Page/Page column 12
![11-(PIPERAZIN-1-YL)DIBENZO[B,F][1,4]THIAZEPINE](/CAS/GIF/5747-48-8.gif)
5747-48-8
90 suppliers
$10.00/1g
![11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE](/CAS/GIF/753475-15-9.gif)
753475-15-9
17 suppliers
$197.00/1mL

110-85-0
560 suppliers
$5.00/5G
![11-Chloro-dibenzo[b,f][1,4]thiazepine](/CAS/GIF/13745-86-3.gif)
13745-86-3
121 suppliers
inquiry
![11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE](/CAS/GIF/753475-15-9.gif)
753475-15-9
17 suppliers
$197.00/1mL