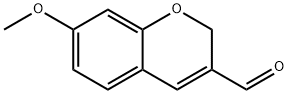
7-METHOXY-2H-CHROMENE-3-CARBALDEHYDE synthesis
- Product Name:7-METHOXY-2H-CHROMENE-3-CARBALDEHYDE
- CAS Number:57543-39-2
- Molecular formula:C11H10O3
- Molecular Weight:190.2
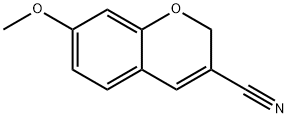
57543-70-1
35 suppliers
inquiry

57543-39-2
31 suppliers
$65.00/100mg
Yield:57543-39-2 93.5%
Reaction Conditions:
with diisobutylaluminium hydride in toluene at -20 - 20; for 4 h;Inert atmosphere;
Steps:
General synthesis of compounds 13a’-13d’
General procedure: To a solution of 13a-13d in toluene (150mg/mL) under the atmosphere of Ar at -20°C, DIBAl-H (1.1eq.) was added. The solution was stirred for 2h at this temperature and another 2h at room temperature. Then acetic ether was (100mL) added and washed with H2O (30 mL) and saline (30 mL) consequently. After the organic solvent was dried over Na2SO4 and removed under vacuum, the resident was purified on a silica gel column to afford the products.
References:
Zhang, Guoning;Liu, Shuainan;Tan, Wenjuan;Verma, Ruchi;Chen, Yuan;Sun, Deyang;Huan, Yi;Jiang, Qian;Wang, Xing;Wang, Na;Xu, Yang;Wong, Chiwai;Shen, Zhufang;Deng, Ruitang;Liu, Jinsong;Zhang, Yanqiao;Fang, Weishuo [European Journal of Medicinal Chemistry,2017,vol. 129,p. 303 - 309] Location in patent:supporting information

673-22-3
473 suppliers
$5.00/1g
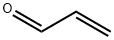
107-02-8
4 suppliers
$38.60/4s8501

57543-39-2
31 suppliers
$65.00/100mg

673-22-3
473 suppliers
$5.00/1g

57543-39-2
31 suppliers
$65.00/100mg