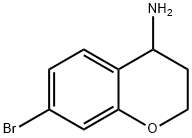
7-broMo-3,4-dihydro-2H-chroMen-4-aMine synthesis
- Product Name:7-broMo-3,4-dihydro-2H-chroMen-4-aMine
- CAS Number:1016804-06-0
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
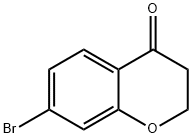
18442-22-3
139 suppliers
$12.00/250mg

1016804-06-0
28 suppliers
$45.00/10mg
Yield:1016804-06-0 77%
Reaction Conditions:
with ammonium acetate;sodium cyanoborohydride in methanol;isopropyl alcohol at 20 - 80; for 16 h;
Steps:
1.1 Step 1. 7-bromo-3,4-dihydro-2H-chroman-4-amine
7-Bromochroman-4-one (10.0 g, 44.04 mmol) was dissolved in a mixed solvent of methanol (100 ml) and isopropanol (125 ml) and then ammonium acetate (67.9 g, 880.9 mmol) and sodium cyanoborohydride (13.84 g, 220.2 mmol) were added thereto at room temperature. The reaction mixture was stirred at room temperature for 4 hours and then refluxed under stirring at 80 for 12 hours. The reaction mixture was cooled to room temperature, concentrated under reduced pressure, basified to pH 8~9 with a 1N sodium hydroxide solution, and then extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate and then filtered and concentrated under reduced pressure. The resulting residue was purified by column chromatography (dichloromethane/methanol = 5/1, v/v) to give the titled compound as a pale yellow oil. (7.8 g, Yield: 77%)
References:
YUHAN CORPORATION;GREEN CROSS CORPORATION WO2021/96241, 2021, A1 Location in patent:Paragraph 458-459
27407-19-8
1 suppliers
inquiry

1016804-06-0
28 suppliers
$45.00/10mg
18385-82-5
29 suppliers
$116.00/1g

1016804-06-0
28 suppliers
$45.00/10mg