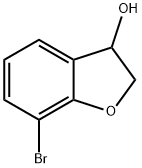
7-Bromo-2,3-dihydrobenzofuran-3-ol synthesis
- Product Name:7-Bromo-2,3-dihydrobenzofuran-3-ol
- CAS Number:1404230-46-1
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
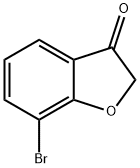
519018-52-1
75 suppliers
$57.00/100mg

1404230-46-1
23 suppliers
inquiry
Yield:1404230-46-1 95 mg
Reaction Conditions:
with chloro([(S,2S)-(?)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido)(mesitylene)ruthenium (II);formic acid;triethylamine in dichloromethane at 20; for 3 h;
Steps:
150.1 Optically active 7-bromo-2,3-dihydrobenzofuran-3-ol
Triethylamine (0.2 ml) was added to formic acid (63 μl), to the resultant mixture, a solution in methylene chloride (2.1 ml) of 7-bromo-2,3-dihydrobenzofuran-3-one (0.10 g) that is commercially available or can be obtained by a publicly known method was added, chloro[(1S,2S)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine]-(mesitylene) ruthenium (II) (8.8 mg) was added, and the resultant mixture was stirred at room temperature for 3 hours. To the resultant mixture, ethyl acetate and water were added, followed by extracting the resultant mixture with ethyl acetate, and the organic phase was washed with a saturated saline, and then was dried over anhydrous sodium sulfate. From the organic phase, the solvent was distilled off under reduced pressure, followed by purifying the resultant residue by silica gel column chromatography (eluate; n-hexane:ethyl acetate=5:1 to 3:1), and from the resultant, the solvent was distilled off under reduced pressure to obtain the subject compound (95 mg) as pale orange oil.
References:
US2012/277150,2012,A1 Location in patent:Paragraph 0951
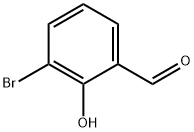
1829-34-1
245 suppliers
$33.00/1g
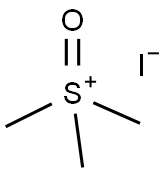
1774-47-6
549 suppliers
$6.00/25g

1404230-46-1
23 suppliers
inquiry